
Energy from Evaporation
July 16, 2015 My first lesson in the thermodynamics of evaporation came from my high school chemistry teacher. As most people know, teacher's salaries aren't all that high. This teacher lived with his family in the rented upstairs apartment of a two-family house, since he couldn't afford his own house. His heating was supplied as part of his rent, but his thermostat temperature adjustment was fixed at a setting too low for his preference. His trick for getting more heat when he felt too cold was to place an alcohol-soaked rag over the thermostat. The evaporation of the alcohol caused a local cooling that tricked the device into giving more heat. The physical mechanism for this is the latent heat of vaporization (also called the enthalpy of vaporization) combined with the temperature change associated with heat capacity. | The Honeywell "round" thermostat. This device, patented as US Patent No. 2,394,920 on February 12, 1946, was sold for many years with a sealed mercury switch activated by a temperature-sensitive bimetallic strip. (Via Wikimedia Commons.) |
 | The drinking bird toy is powered by the evaporation of water. Evaporation of water from the felt-covered beak causes dichloromethane to condense because of the lowered temperature. (Via Wikimedia Commons.) |
"Evaporation is a fundamental force of nature... It's everywhere, and it's more powerful than other forces like wind and waves... Our climate is powered by evaporating water from the oceans, and we have no direct way of accessing this energy."[3,6]There's been previous research on materials that respond mechanically to chemical stimuli, and there have also been biomimetic systems that oscillate, transport fluid, and change shape. These materials have been far less efficient at generating work compared with conventional actuators.[5] Last year, researchers from Columbia University, Harvard University, and Loyola University teamed in a demonstration that Bacillus spores exert a mechanical response of 10 MJ/m3 to water gradients.[5] This is a thousand times more force than human muscles and a hundred times greater than synthetic water-responsive materials.[5-6]
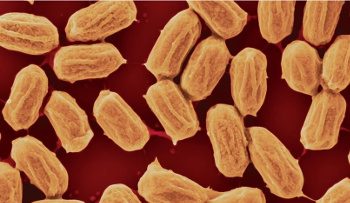 | Bacillus bacterial spores. Bacillus bacteria are a common soil microorganism. (Still image from a YouTube Video.)[] |
"Changing size this much is highly unusual for a material that is as rigid as wood or plastic... We figured that expanding and contracting spores can act like a muscle, pushing and pulling other objects. We noticed that we could harness the motion of spores and convert it to electrical energy."[6]In last year's study, the research team identified mutations that almost double the energy density of the spores. They were also able to get the spores to self-assemble into dense, submicrometer-thick monolayers on various substrates, including sheets of elastomer and silicon microcantilevers.[5] In that first study, the research team demonstrated the utility of their material by building an energy-harvesting device in which movement of a flexing membrane creates electricity by moving a magnet inside a coil of wire (see figure).[5]
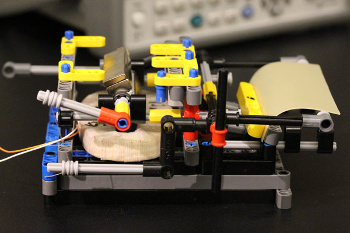 | Even scientists play with Lego®! In this device, a spore-coated rubber sheet moves a magnet inside a coil of wire to produce electricity. (Columbia University image by Xi Chen.) |
 | The evaporation-powered car, called "Eva," is propelled by a turbine engine fueled by water evaporating from wet paper. (Columbia University image by Xi Chen.) |
 | The Evaporation Engine floats on water, and its spore mechanism creates a piston movement capable of generating electricity. (Columbia University image by Xi Chen.) |
References:
- Data Page for Isopropyl Alcohol, NIST.
- Xi Chen, Davis Goodnight, Zhenghan Gao, Ahmet H. Cavusoglu, Nina Sabharwal, Michael DeLay, Adam Driks, and Ozgur Sahin, "Scaling up nanoscale water-driven energy conversion into evaporation-driven engines and generators," Nature Communications, vol. 6, article no. 7346 (June 16, 2015), doi:10.1038/ncomms8346. This is an open access publication with a PDF file available here.
- Renewable energy from evaporating water, Columbia University Press Release, June 16, 2015.
- Renewable Energy from Evaporating Water, YouTube Video by ExtremeBio, June 16, 2015.
- Xi Chen, L. Mahadevan, Adam Driks, and Ozgur Sahin, "Bacillus spores as building blocks for stimuli-responsive materials and nanogenerators," Nature Nanotechnology, vol. 9, no. 2 (2014), http://dx.doi.org/10.1038/nnano.2013.290.
- Beth Kwon, "Biophysicist Harnesses Power of Evaporation, Discovers Potential New Source of Renewable Energy," Columbia University Press Release, January 28, 2014.
- Bacterial Spores Harness Evaporation Energy, YouTube Video by extremebio.org, January 27, 2014.
- Extremebio.org Web Site.