Bouncing Droplets
December 3, 2015
An occasional
theme in early
cinema was
dripping water keeping someone awake. In the
cartoon world, a
hibernating bear was the most likely victim. Dripping water would keep me awake some nights, not because I could hear it in the
bedroom, but because I knew I would need to fix a leaky
water faucet. I do most of my own
plumbing; and, as a rule, I find that all plumbing jobs escalate to a much larger task than expected, just like
software development.
The
United States has benefited from abundant
water for
agriculture and personal use, but times have changed. For the past four years there's been a severe
drought in the
Colorado River basin, and
California, which uses a third of that
river's flow, has been greatly affected.[1] This exceptional drought affects not just some
western states, but all states that receive agricultural goods from these areas. California has been exporting "
virtual water" out of the state at an unsustainable rate.
Virtual water is water used in the
growth,
harvesting and
packaging of
food, or the
manufacture of
commodities. I wrote about the concept of virtual water in two previous articles,
Virtual Water, March 4, 2013 and
Virtual Water in the Roman Empire, January 22, 2015. California exports large quantities of virtual water not just through agriculture, but in
electronics. It takes
gallons of water to produce a single
integrated circuit.
According to
Mother Jones, it might be better from a conservation of virtual water standpoint to have
wine rather than
walnut muesli for
breakfast. The following table shows how many
gallons of virtual water are contained in the following
produce items.[2]
I mentioned fixing leaky faucets in my house. While water drops don't seem to amount to much, the
U.S. Environmental Protection Agency estimates that water leaks in a typical
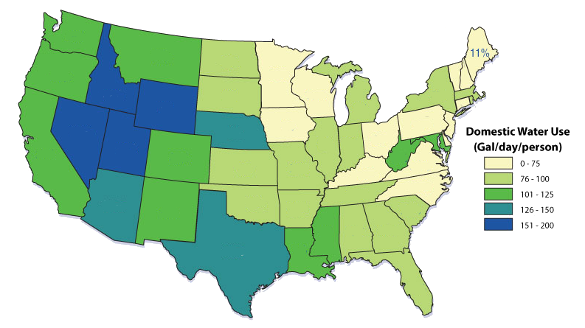
household waste more than 10,000 gallons of water annually.[3] This amounts to more than a trillion (million-million) gallons wasted annually nationwide. One out of every ten homes have leaks wasting 90 gallons or more per day.[3] The following map shows current water usage across the United States.[4]
While drips and drops seem quite ordinary, there's a lot of
physics behind the formation, movement, and impact of droplets. I've written several articles about droplets, including the
technologically important processes of
hydrophilicity and
hydrophobicity (
Evaporation, August 7, 2013,
Icing-Resistant Surfaces, August 8, 2012,
Superhydrophobic Anti-Glare Glass, May 2, 2012, and
Tiny Droplets, March 6, 2012).
Mechanical engineers from the
Swiss Federal Institute of Technology (ETH, Zurich, Switzerland) have just discovered an unusual
phenomenon associated with water droplets on
superhydrophobic textured surfaces in
low-pressure environments.[5-6]
Hydrophobicity, in which a surface resists wetting, is technologically important. Hydrophobic surfaces that resists
icing are important for
aviation, and for
power transmission lines. The ETH research team discovered that a particular
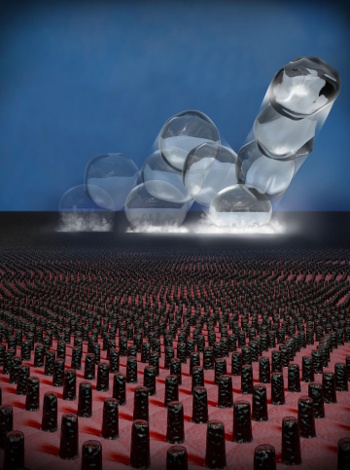
microstructuring creates a superhydrophobic surface that will self remove water droplets in a low-pressure environment. The droplets suddenly levitate and bounce off the surface in a
trampoline-like bouncing behavior. Sequential collisions with the surface
accelerate the droplets until they bounce away.[5-6]
In the ETH
experiments, one
millimeter-sized water droplets were placed on a rigid, microstructured,
silicon surface and the air pressure was reduced. Using a
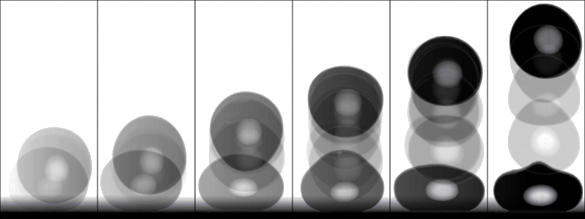
high speed camera to monitor these droplets, it was found that they first remain motionless on the surface; then, at about a twentieth of
atmospheric pressure, they suddenly jump up. When they land again on the surface, they jump again, only to a higher height.[6]
Such behavior is confusing. While the rigid silicon surface can't provide any additional energy to the droplets, the
restitution coefficient, the
ratio of speed after the collision of before the collision is greater than one, an apparent violation of the
second law of thermodynamics.[5-6] Of course, experiment showed that no
perpetual motion is involved.
Vapor pressure from
evaporation of the droplet, contained by voids in the surface texture and the initial
adhesion of the droplet, launches the droplet upwards at each impact.[5-6]
If the evaporating water
supercools below its
freezing point and
ice crystals form, the
latent heat of fusion will
heat the droplet to the freezing point in a few
milliseconds, leading to explosive evaporation, and the droplet shoots up like a
rocket.[6] This effect lifts icy drops the moment that they freeze.[5]
The process was
optimized by arranging the microstructure to have
columns a few
micrometers wide spaced about five micrometers apart. The surface needs to be
rough enough to be superhydrophobic, but not so rough as to let the water vapor escape too quickly through the surface channels.[6] Says ETH
professor,
Dimos Poulikakos,
"From the results of our research we can deduce what qualities surfaces need to have in general in order for them to violently repel water and ice, and then design them accordingly."[6]
These observations are for droplets in a low-pressure environment, so it remains to be seen how this could be useful in real-world applications.[5-6]
References:
- Abrahm Lustgarten, Lauren Kirchner, Amanda Zamora and ProPublica, Scientific American, "America's water crisis is so much bigger than California," Salon, June 27, 2015.
- Alex Park and Julia Lurie, "It Takes How Much Water to Grow an Almond?!" Mother Jones, February 24, 2014.
- WaterSense - Fix a Leak Week, US Environmental Protection Agency, October 16, 2016.
- WaterSense - Our Water - Tomorrow & Beyond, US Environmental Protection Agency, October 16, 2016.
- Thomas M. Schutzius, Stefan Jung, Tanmoy Maitra, Gustav Graeber, Moritz Köhme, and Dimos Poulikakos, "Spontaneous droplet trampolining on rigid superhydrophobic surfaces," Nature, vol. 527, no. 7576 (November 5, 2015), pp. 82-85, doi:10.1038/nature15738.
- Trampolining water droplets, Eidgenössische Technische Hochschule Zürich Press release, November 4, 2015.