
Bouncing Batteries
May 14, 2015 Today, entertainment is more likely enjoyed in a personal, rather than a public, setting. For music, we apply earpieces instead of traveling to the concert hall. For movies, we stare at the small display screen of our cellphone and not the wide screen of the movie theater. Instead of interacting with a machine, audiences of the past would sometimes interact with each other; and, in Shakespeare's time, they might even interact with the actors by throwing ripe fruit. Long before The Rocky Horror Picture Show, people would participate in public performances by singing along with the "bouncing ball." As a song was sung on screen, the lyrics would be displayed with a white ball bouncing from word-to-word to encourage the audience to sing along. This effect originated in a 1925 cartoon version of My Bonnie Lies over the Ocean. To view a cartoon version of Little Brown Jug, see ref. 1.[1] | A ball of a different sort. Buckminsterfullerene, C60, also called a bucky-ball. It's named after Buckminster Fuller, popularizer of the similarly constructed geodesic dome. (Modified Wikimedia Commons image by Mstroeck.) |
 |
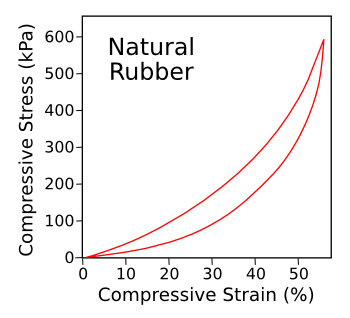 | Hysteresis curve for natural rubber. The area inside the curve corresponds to the energy loss in the cycle. (Illustration by the author using Inkscape.) |
"A year ago a buddy of mine who knows I work on this sent me this video and said did you know this happens? I didn't. But I had a bunch of batteries on my desk and I was able to verify it... The bounce does not tell you whether the battery is dead or not, it just tells you whether the battery is fresh."[6]Steingart's research team built a simple apparatus that uses a computer microphone to record the sound made when a battery falls through a plexiglass tube. The time between bounces was used to determine the height of the bounce.[6] The simplicity of the experiment makes its results readily accessible to people without a scientific background (see video, below).[6]
 | Bouncing batteries, demonstrating the non-linear discharge effect. (Princeton University video.) |
"The zinc starts out as a packed bed of particles that all move very nicely past each other... When you oxidize the zinc, it makes bridges between the particles and makes it more like a network of springs. That is what gives the battery its bounce."[6]The coefficient of restitution was found to correlate with the formation of these zinc oxide bridges in the zinc anode, and it was found to level off at a value of 0.66 ± 0.02 at 50% discharge, the point at which the anode has densified into a porous zinc oxide solid.[5] It's interesting that zinc oxide is added to golf balls to give them extra bounce.[6] This research was funded by the National Science Foundation, the U.S. Department of Energy, and Brookhaven National Laboratory.[6] Since I've mentioned the coefficient of restitution, I would be remiss in not mentioning the super bouncy Super Balls and bouncy balls. The synthetic rubber material of the Super Ball was invented by chemist, Norman Stingley, in 1964 and patented in 1966.[7] The material is a polybutadiene matrix containing zinc oxide and stearic acid, among other ingredients.[7] It's vulcanized with sulfur at high temperature and pressure, and the resulting balls have a coefficient of restitution from 0.9 to nearly 1.0.
References:
- Little Brown Jug (Cartoon Sing along), YouTube Video, September 26, 2007.
- Howard Brody, "The tennis‐ball bounce test," Phys. Teach., vol. 28, no. 6 (September, 1990) pp. 407ff., http://dx.doi.org/10.1119/1.2343088. A PDF version of this article can be found here.
- Ball Testing, Tennis Industry Magazine, July, 2007.
- Lee Hite, "Why A Dead Alkaline Battery Bounces!" YouTube Video, December 27, 2013.
- Shoham Bhadra, Benjamin J. Hertzberg, Andrew G. Hsieh, Mark Croft, Joshua W. Gallaway, Barry J. Van Tassell, Mylad Chamoun, Can Erdonmez, Zhong Zhong, Tal Sholklapperh, and Daniel A. Steingart, "The relationship between coefficient of restitution and state of charge of zinc alkaline primary LR6 batteries," Journal of Materials Chemistry A, Advance Article, March 13, 2015, DOI: 10.1039/C5TA01576F.
- John Sullivan, "Battery bounce test often bounces off target," Princeton University Press Release, March 20, 2015.
- Norman H Stingley, "Highly resilient polybutadiene ball," U.S. Patent No. 3,241,834, March 22, 1966.