
Strange Salt
January 15, 2014 Sodium chloride (table salt, NaCl) is a chemical compound that appears in the very early chapters of nearly every chemistry textbook. Sodium, which has atomic number 11, and chlorine, which has atomic number 17, appear very early in the Periodic Table, in the third period. As a group 1 metal, sodium has a single positive charge when it's ionized by losing an electron (Na+). As a group 17 halogen, chlorine accepts an electron to become an ion with a single negative charge (Cl-). When these ions get together, it's easy to see how the stable compound, NaCl, will form. The crystal form of NaCl is called halite, and that's how the halogens ("salt makers") get their name. The crystal structure of halite is the aptly named rock salt structure, which is a face-centered cubic crystal (see figure).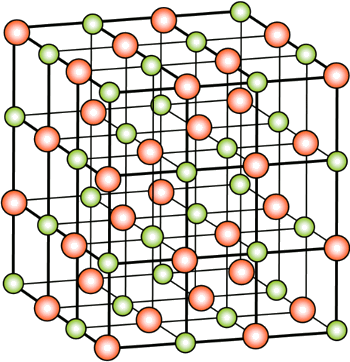 | Crystal structure of rock salt (NaCl, halite) (Image by H. Hoffmeister, via Wikimedia Commons.) |
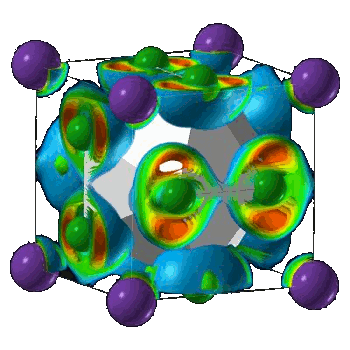 | Crystal structure of NaCl3. (Illustration by Artem Oganov, from Carnegie Institution press release.) |
"These compounds are thermodynamically stable and once made, remain so indefinitely... Classical chemistry forbids their very existence. Classical chemistry also says atoms try to fulfill the octet rule - elements gain or lose electrons to attain an electron configuration of the nearest noble gas, with complete outer electron shells that make them very stable. Well, here that rule is not satisfied."[5-6]Alexander Goncharov of the Carnegie Institution sees the possibility for future work.
"If this simple system is capable of turning into such a diverse array of compounds under high-pressure conditions, then others likely are, too... This could help answer outstanding questions about early planet cores, as well as to create new materials with practical uses."[4]This work was supported in the US by the National Science Foundation, the Army Research Office and DARPA, among other institutions.[4]
References:
- J. S. Schilling, "What High Pressure Studies Have Taught Us About High-Temperature Superconductivity," Frontiers of High Pressure Research II: Application of High Pressure to Low-Dimensional Novel Electronic Materials (Kluwer Academic Publishers, 2001), pp. 345-360 (PDF file).
- Weiwei Zhang, Artem R. Oganov, Alexander F. Goncharov, Qiang Zhu, Salah Eddine Boulfelfel, Andriy O. Lyakhov, Elissaios Stavrou, Maddury Somayazulu, Vitali B. Prakapenka and Zuzana Konôpková, "Unexpected Stable Stoichiometries of Sodium Chlorides," Science, vol. 342, no. 6165 (December 20, 2013), pp. 1502-1505.
- Jordi Ibáñez Insa, Perspective - Geochemistry-Reformulating Table Salt Under Pressure, Science, vol. 342, no. 6165 (December 20, 2013), pp. 1459-1460.
- Throwing out the textbook: salt surprises chemists, Carnegie Science Press Release, December 19, 2013.
- Salzige Überraschung - Forscher finden "verbotene" Verbindungen von gewöhnlichem Kochsalz, Deutsches Elektronen-Synchrotron Press Release, December 19, 2013 (in German).
- Salty surprise -- ordinary table salt turns into 'forbidden' forms, Deutsches Elektronen-Synchrotron Press Release, December 19, 2013 (in English).
- Weiwei Zhang, Artem R. Oganov, Alexander F. Goncharov, Qiang Zhu, Salah Eddine Boulfelfel, Andriy O. Lyakhov, Elissaios Stavrou, Maddury Somayazulu, Vitali B. Prakapenka and Zuzana Konôpková, "Unexpected Stable Stoichiometries of Sodium Chlorides," Science, vol. 342, no. 6165 (December 20, 2013), pp. 1502-1505.