
Topological Insulators and Stanene
February 24, 2013 The name of physicist, Enrico Fermi, is attached to many things, one of these being the Fermi level. The Fermi level, often symbolized as μ, is the energy level that would be occupied by electrons half the time at thermodynamic equilibrium. The probability f(ε) of an electron being at any particular energy level, ε, at temperature, T, is given by the Fermi-Dirac distribution,where k is the Boltzmann constant. It's easy to see from this equation that a probability of one-half is obtained when the energy is the Fermi level energy; that is, when ε-μ = 0). There's no requirement that a solid must have electrons at the Fermi level. It just describes how electrons at that level would behave. In the band theory of solids, the location of the Fermi level with respect to the energy bands of a material determines the material's electrical conductivity. Insulators have a large band gap between the energy of their valence electrons, residing in a valence band, and conduction electrons, residing in a conduction band. The Fermi level sits within this gap, and there are few electrons with sufficient energy to jump the gap. In a metal, the Fermi level is within the conduction band, so there are electrons available for conduction. In semiconductors, the Fermi level is outside the conduction band, but it's close enough for electrons to be excited into the conduction band to allow some conduction. This tutorial is prologue to a description of an interesting type of insulator, the topological insulator.[1-3] As the figure shows, these have a Fermi level between the valence band and the conduction band, as in other insulators. However, their surface electrons have a different trajectory in energy-momentum space, so these materials are bulk insulators and surface conductors at the same time.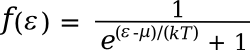
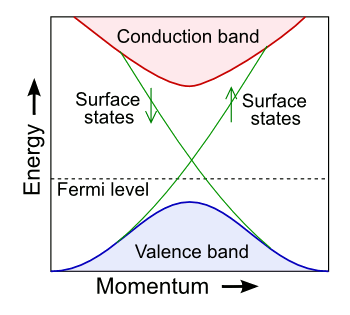 | Simplified band structure of a topological insulator. (Modified image by "A13ean," via Wikimedia Commons.) |
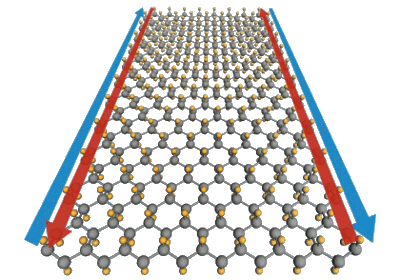 | In this illustration, tin atoms (gray) are decorated with fluorine anions (yellow). Fluorine is predicted to make this a superconducting topological insulator. (Yong Xu/Tsinghua University; Greg Stewart/SLAC.) |
"Stanene could increase the speed and lower the power needs of future generations of computer chips, if our prediction is confirmed by experiments that are underway in several laboratories around the world... Eventually, we can imagine stanene being used for many more circuit structures, including replacing silicon in the hearts of transistors... Someday we might even call this area Tin Valley rather than Silicon Valley."[5]
References:
- Charles Kane and Joel Moore, "Topological insulators," Physics World, February, 2011, pp. 32-36 (PDF file).
- Michel Fruchart and David Carpentier, "An Introduction to Topological Insulators," arXiv Preprint Server, November 3, 2013. Available, also, as Michel Fruchart and David Carpentier, "An Introduction to Topological Insulators," Comptes Rendus Physique, vol. 14, nos. 9-10 (November–December, 2013), pp. 779-815.
- Ali Yazdani, "Topological Insulators - Overview," Princeton University Web Site.
- Yong Xu, Binghai Yan, Hai-Jun Zhang, Jing Wang, Gang Xu, Peizhe Tang, Wenhui Duan and Shou-Cheng Zhang, "Large-Gap Quantum Spin Hall Insulators in Tin Films," Physical Review Letters, vol. 111, no. 13 (September 27, 2013), Document No. 136804 [5 pages] . Available, also, from arXiv.
- Will 2-D Tin be the Next Super Material?, SLAC Press Release, November 21, 2013.
- Will 2D tin be the next super material for chip interconnects?, Kurzweil Accelerating Intelligence, November 25, 2013.
- Charles Q. Choi, "Could Atomically Thin Tin Transform Electronics?," Scientific American, December 4, 2013.
- Rik Myslewski, "OHM MY GOD! Move over graphene, here comes '100% PERFECT' stanene," The Register (UK), December 4, 2013.
- Michel Fruchart and David Carpentier, "An Introduction to Topological Insulators," arXiv Preprint Server, November 3, 2013. Available, also, as Michel Fruchart and David Carpentier, "An Introduction to Topological Insulators," Comptes Rendus Physique, vol. 14, nos. 9-10 (November–December, 2013), pp. 779-815.