
Crystals without Facets
November 7, 2014 I was born and raised in Upstate New York, about fifteen miles from Herkimer, New York, a village with a population of less than 10,000 people. One summer, I tended to the transmitter of a small AM radio station there while its chief engineer was on vacation. The Herkimer area is famous for Herkimer diamonds. These are large, clear crystals found in dolomite rock, and they aren't really diamonds. They're quartz, the crystalline form of silicon dioxide (silica, SiO2), and they're impressive since they are large and double-terminated; that is, they exhibit facets all around.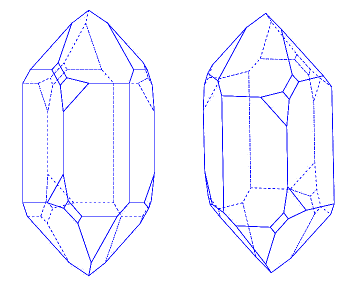 | Crystal facets of quartz. Quartz grows as left- and right-handed crystals, as shown. (Left image and right image by "strickja," via Via Wikimedia Commons, modified.) |
 | Synthetic Berlinite (AlPO4), crystals grown by hydrothermal synthesis.[3] The small divisions on the ruler are millimeters. This material is isostructural with quartz. (Photo by the author, as uploaded to Wikimedia Commons.) |
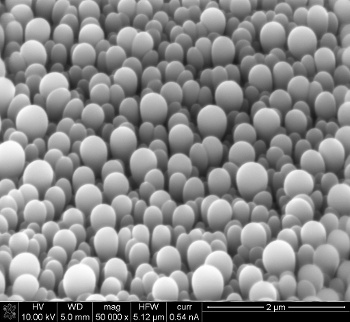 | Micrograph of the non-faceted texture of a boron subphthalocyanine chloride layer. (Image: University of Michigan/Max Shtein.) |
"In my years of working with these kinds of materials, I've never seen shapes that looked like these. They're reminiscent of what you get from biological processes... Nature can sometimes produce crystals that are smooth, but engineers haven't been able to do it reliably."[6]Boron subphthalocyanine chloride, from which these layers are made, is a small molecule that makes either flat films or faceted crystals with sharp edges. Once again, serendipity advanced science when Shaurjo Biswas, a doctoral student at the University of Michigan in 2010, was using organic vapor jet printing to make solar cells from this material. He found that layers of thickness greater than about 600 nm exhibited a texture, so he decided to make thicker films. The nanolobe pattern emerged at a thickness of 800 nm.[6] After a while, Shalev continued production and analysis of these layers, systematically varying the growth conditions in an improved apparatus. Eventually, others joined the research effort that culminated in a paper in a recent issue ofNature Communications.[6] Shtein had helped to develop the organic vapor jet printing process as a graduate student. It's essentially like spray painting, but with a gas instead of a liquid, and it has the advantage that the process doesn't require a vacuum.[4,6]
 | Portion of the organic vapor jet printing apparatus. This photo conveys no useful information, but I always enjoy looking at laboratory apparatus. (Still image from a YouTube video.)[7] |
References:
- A. C. Walker, "Hydrothermal Synthesis of Quartz Crystals," Journal of the American Ceramic Society, vol. 36, no. 8 (August, 1953), pp. 250-256.
- Ernest Buehler, "Method of growing quartz crystals," US Patent No. 2,785,058, March 12, 1957.
- Bruce H. Chai, Ernest Buehler, and John J. Flynn, "Alpha aluminum- or alpha gallium- orthophosphate crystals, wafers for acoustic wave devices," US Patent No. 4,559,208, December 17, 1985.
- O. Shalev, S. Biswas, Y. Yang, T. Eddir, W. Lu, R. Clarke, and M. Shtein, "Growth and modelling of spherical crystalline morphologies of molecular materials," Nature Communications, vol. 5, article no. 5204 (October 16, 2014), doi:10.1038/ncomms6204.
- Supplementary information for ref. 4 (PDF File).
- Nicole Casal Moore, "Facetless crystals that mimic starfish shells could advance 3D-printing pills," University of Michigan Press Release, October 20, 2014.
- Nanolobes, YouTube video by Michigan Engineering, October 16, 2014.