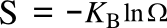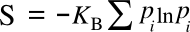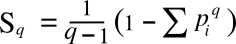Tsallis Entropy
October 29, 2014
Thermodynamics has developed from contributions from
scientists of many
nationalities. As just a few examples, we have
Sadi Carno (1796-1832) from France;
Robert Boyle (1627-1691) and
William Thomson, Lord Kelvin (1824-1907) from Ireland;
James Prescott Joule (1818-1889) from England;
Rudolf Clausius (1822-1888) from Germany;
James Clerk Maxwell (1831-1879) from Scotland;
Josiah Willard Gibbs (1839-1903) from the United States; and
Ludwig Boltzmann (1844-1906) from Austria).
 |
| Some founders of thermodynamics. Clockwise from the upper left, Carno, Boyle, Kelvin, Joule, Boltzmann, Gibbs, Maxwell, and Clausius. Images, via Wikimedia Commons, Carnot, Boyle, Kelvin, Joule, Clausius, Maxwell, Gibbs, Boltzmann. (Click for larger image). |
It's only fitting that
Greece, the origin of the
Western intellectual tradition of
philosophy and
science, should also have been the birthplace of some prominent
thermodynamicists. One of these is
Constantin Carathéodory (1873-1950), whose
profession was primarily
mathematics.
In 1909, Carathéodory ventured into
applied mathematics by working to formulate thermodynamics
axiomatically. His book, "Investigations on the Foundations of Thermodynamics," sought to derive thermodynamics from
mechanics. The importance of this approach is that he was able address
irreversibility. Classical thermodynamics is accurately applied only to
reversible processes, although bending of this rule still leads to acceptable results.
Carathéodory's work is somewhat inaccessible because of all the mathematics, but another Greek thermodynamicist developed a modification of the classical
Boltzmann entropy that's easy to understand.
Constantino Tsallis (b. 1943) is a
physicist who's a
naturalized citizen of
Brazil, but he was born in
Athens, Greece, lived in
Argentina, and he was awarded his
doctotal degree in
physics from the
University of Paris-Orsay.
Everyone who has taken a few physics or
chemistry courses is familiar with
Boltzmann's entropy formula,
in which
S is the
entropy,
KB is the
Boltzmann constant (1.38062 x 10
-23 joule/
kelvin), and
Ω is the number of states accessible to the system. This equation is actually a simplification of the
Boltzmann-Gibbs entropy when every state has the same
probability pi; viz,
These equations define a maximum entropy, since the equal probability means that you can stuff an
atom or
molecule into any accessible state.
At this point, we might wonder what will happen when some states are
correlated with each other, a condition for which the entropy will be less than the maximum. That's what Tsallis did in a 1988 paper[1] in which he defined what's now known as the
Tsallis entropy,
Sq, given by the following equation:
in which the
parameter,
q, is called the entropic-index. This equation becomes the Boltzmann-Gibbs entropy equation when we take the
limit as q approaches one. It's been a while since I did limits in
Calculus, so I'll just take this on faith.
There are presently 69
papers posted on
arXiv having "Tsallis entropy" in their titles. What's so important about Tsallis entropy? Boltzmann entropy applies only to
systems in equilibrium, and the Boltzmann entropy is an
extensive function; that is, it depends on how much
matter we have in our system. In non-equilibrium systems we need another way to look at entropy, and now we have Tsallis entropy, which is non-extensive. As can be imagined, a lot of people have a problem with a non-extensive entropy.
Tsallis entropy has been shown to produce better results than Boltzmann entropy for the analysis of some systems in
biology,
nuclear physics,
finance,
music, and
linguistics; and the correlations in
DNA sequences.[2] Despite these successes, there's been criticism that the entropic-index
q acts merely as a
fitting parameter. There's also an argument that Tsallis entropy violates the
zeroth law of thermodynamics. That's the law that states that systems in thermal equilibrium with another system are in equilibrium with each other.[3]
In the end, it appears that
physicists just need to know whether to apply one entropy or another to a given system. It's not unlike knowing that you should use
classical mechanics for
bowling balls and
quantum mechanics for atoms.[3]
References:
- Constantino Tsallis, "Possible generalization of Boltzmann-Gibbs statistics," Journal of Statistical Physics, vol. 52, nos. 1-2 (July, 1988), pp. 479-487.
- H. V. Ribeiro, E. K. Lenzi, R. S. Mendes, G. A. Mendes, and L. R. da Silva, "Symbolic Sequences and Tsallis Entropy," arXiv Preprint Server, January 16, 2014.
- Jon Cartwright, "Roll over, Boltzmann," Physics World, May, 2014, pp. 31-35.