
Whiskers
March 22, 2013 For a brief span in my career, I did research in the growth of technologically useful crystals. Crystals grow in different shapes, with facets representative of their underlying atomic structure. Sometimes, the growth rate along certain crystal directions can be quite a bit larger than others, so your crystal becomes long and extended. Adding impurities to certain crystallizing chemicals can change the resulting crystal product from an expected geometrical object to a long whisker. scientists employed even when industrial processes have been working without change for many years and their scientists seem redundant. In a previous article (Tin Pest and Purple Plague, April 24, 2012), I wrote how some solder alloy compositions designed to replace leaded solder were prone to formation of whiskers. The problem started with the European Restriction of Hazardous Substances Directive (RoHS), and directives in other countries, for removal of hazardous chemicals from products, including lead in electronic devices. The eutectic composition of the lead-tin binary alloy system has been used as an electronic solder for more than a century. The reasons for this are its low melting point (183 °C), low cost, and good electrical conductivity. One lead-free option is to use pure tin (melting point, 232 °C), but tin will form tin whiskers during repeated thermal cycling, the presence of which can lead to electrical short circuits if they dislodge from the solder ball and are deposited elsewhere in the circuitry.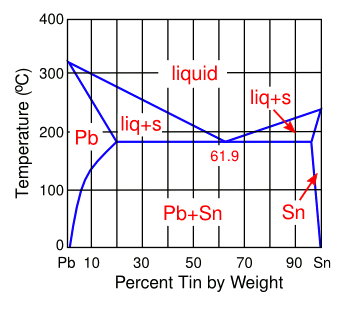 | Lead-Tin (Pb-Sn) Phase Diagram The eutectic composition, at 61.9 wt-% tin, melts at 183 °C, quite a bit lower than the melting point of tin (232 °C). (Author's rendition using Inkscape.) |
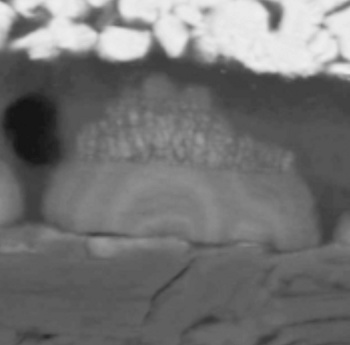 | Scanning electron micrograph of a lithium dendrite cross-section showing a ring structure. Each ring is associated with a discharge-charge cycle. (Purdue University Image by Quinn Horn.) |
References:
- Yong Sun, Elizabeth N. Hoffman, Poh-Sang Lam and Xiaodong Li, "Evaluation of local strain evolution from metallic whisker formation," Scripta Materialia, vol. 65, no. 5 (September, 2011), pp. 388-391.
- David R. Elyz and R. Edwin Garcıa, "Heterogeneous Nucleation and Growth of Lithium Electrodeposits on Negative Electrodes," J. Electrochem. Soc., vol. 160, no. 4 (February 12, 2013), pp. A662-A668.
- Analytical theory may bring improvements to lithium-ion batteries dendrites, Purdue University Press Release, March 5, 2013.
- David R. Elyz and R. Edwin Garcıa, "Heterogeneous Nucleation and Growth of Lithium Electrodeposits on Negative Electrodes," J. Electrochem. Soc., vol. 160, no. 4 (February 12, 2013), pp. A662-A668.
