
Persistent Photoconductivity
November 27, 2013 Selenium would have been an uncommon material were it not for two electrical properties discovered in the very early days of electronics. The most useful of these is its ability to act as a type of rectifier called a metal rectifier. This rectifier action (changing alternating current to direct current) was discovered by Charles Fritts, circa 1886, but such rectifiers were only made practical in the 1930s. The selenium rectifier elements, looking like the fins of today's semiconductor heat sinks (see photograph), were aluminum or steel plates, coated with a thin layer of bismuth or nickel, and then coated with a thick (~0.0025 inch) layer of selenium. Heat treatment ensured conversion of the selenium to its gray, hexagonal phase.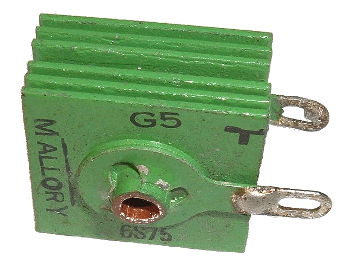 | A selenium rectifier with inch square plates. Selenium rectifiers were common when I first started experimenting with electronics in the early 1960s. (Photograph by Arnold Reinhold, viaWikimedia Commons.) |
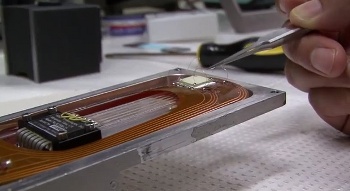 | Mounting a strontium titanate specimen (white square) for measurement. (Still image from a YouTube video.[8]) |
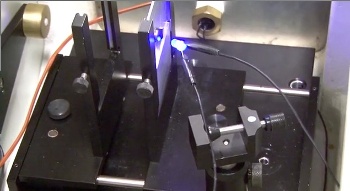 | Measuring photoconductivity of a strontium titanate specimen. (Still image from a YouTube video.[8]) |
“The discovery of this effect at room temperature opens up new possibilities for practical devices. In standard computer memory, information is stored on the surface of a computer chip or hard drive. A device using persistent photoconductivity, however, could store information throughout the entire volume of a crystal.”[7]The Washington State University research was funded by the National Science Foundation.[7]
References:
- "Effect of Light on Selenium during the passage of an Electric Current" (W. Smith), Nature, vol. 7, no. 173 (February 20, 1873), pp. 303-303.
- W. G. Adams and R. E. Day, "The Action of Light on Selenium," Proc. R. Soc. Lond., vol. 25 (January 1, 1876), pp. 113-117, doi:10.1098/rspl.1876.0024 (PDF File).
- C. E. Fritts, "On a new form of selenium cell, and some electrical discoveries made by its use," American Journal of Science, vol. 26, series 3 (December, 1883), pp. 465-472.
- Chester F Carlson, "Electrophotography," US Patent No. 2,297,691, October 6, 1942.
- Leon Merker, "Refractive material," US Patent No. 2,764,490, September 25, 1956.
- Farida A. Selim and Matthew D. McCluskey, "Persistent Photoconductivity in Strontium Titanate," Physical Review Letters, vol. 111, no. 18 (November 1, 2013), Document No. 187403 [5 pages].
- Accidental discovery dramatically improves conductivity, Washington State University Press Release, November 14, 2013.
- Washington State University, "Accidental Discovery Dramatically Improves Electrical Conductivity," YouTube Video, November 15, 2013.
- W. G. Adams and R. E. Day, "The Action of Light on Selenium," Proc. R. Soc. Lond., vol. 25 (January 1, 1876), pp. 113-117, doi:10.1098/rspl.1876.0024 (PDF File).