
Storing Heat
March 29, 2013 Many people where I live in Northern New Jersey have the same wish about climate; surprisingly, it doesn't concern global warming. All of us would like to store the oppressive heat of our summer so it can be used in the winter. Twenty-three degrees of angle doesn't sound like much, but this tilt of Earth's axis with respect to its orbital plane causes the insolation to change over the course of the year, transforming degrees of angle to degrees of temperature. When I was a child, my father would take his sons fishing at a large, secluded pond in Upstate New York. Few people knew about this pond; but, many decades prior, it was an industrial site for storing the cold of winter for use in the summer. There was an ice house on the shore, and the pond ice was cut and stored under mounds of sawdust insulation. Residential iceboxes used the phase change of ice to water to extract heat energy from food, thereby keeping the food cool. I reviewed the use of phase change to store energy in a previous article (Solar Salt, July 30, 2010). The Archimede solar energy plant near Syracuse, Sicily, uses the latent heat of fusion (also called the enthalpy of fusion) of inexpensive nitrates to store solar energy. One particular mixture of nitrates, (NaNO3)7(KNO3)44(NaNO3)49 has a liquidus temperature of 141°C and a latent heat of fusion of 132.5 kJ/kg. This mixture, called Hitec®, is sold by Coastal Chemical Co., L.L.C., Houston, Texas.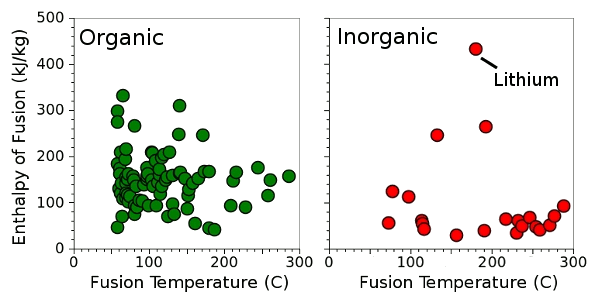 |
| Enthalpy of fusion for 83 organic and 31 inorganic chemicals with melting points below 300°C. (Data collected by the author from various sources, graphed using Gnumeric.) |
 | Slabs of paraffin, such as this, were common in kitchens of yesteryear. Molten food-grade paraffin was used to seal jars of home-made jellies and jams. (Via Wikimedia Commons.) |
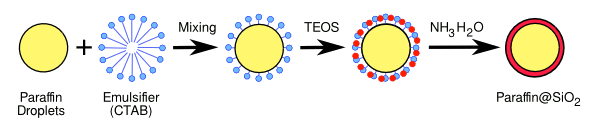 |
| Process for encapsulation of microdroplets of paraffin in silica. Illustration by the author using Inkscape. |
References:
- Benxia Li, Tongxuan Liu, Luyang Hu, Yanfen Wang and Lina Gao, "Fabrication and Properties of Microencapsulated Paraffin@SiO2 Phase Change Composite for Thermal Energy Storage," ACS Sustainable Chem. Eng., vol. 1, no. 3 (2013), pp 374-380.
- Paraffin encapsulated in beach sand material as a new way to store heat from the sun, American Chemical Society Press Release, March 13, 2013.