
Ocean Manganese
September 20, 2013 When I was in graduate school in the early 1970s, there was a lot of press about mining sea floor manganese nodules. These nodules are so-named because they are mostly manganese (27-30%), although they contain quite a number of other useful metals, such as nickel (1.25-1.5%), copper (1-1.4%) and cobalt (0.2-0.25%). They are sometimes called polymetallic nodules, since they incorporate these and other metals as well. I wrote about these nodules in a previous article (The Manganese Conspiracy, June 9, 2010). | Sea floor manganese nodules. (United States Geological Survey photograph, via Wikimedia Commons.) |
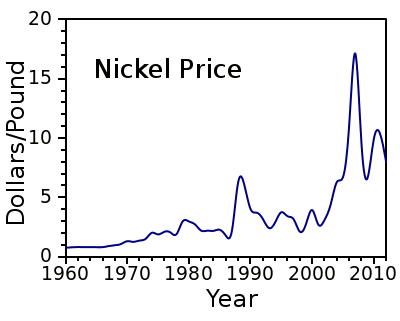 | The price of nickel, 1960-2012, from US Geological Survey data.[1-2] (Plot by author, using Gnumeric.) |
 | The Hughes Glomar Explorer (Via Wikimedia Commons.) |
"You wouldn't think manganese is that important, but without manganese, we wouldn't have the molecular oxygen that we breathe."[4]Manganese has several oxidation states, and the research team found that oceanic manganese(III) is far more prevalent than previously thought.[4] In previous studies of this element, only the total dissolved manganese was measured, and it was presumed that it was all manganese(II).[3-4] The first indication that Mn(III) might be prevalent was a study by Luther in the mid-2000s in which this oxidation state was found in Black Sea waters in which a gradient in oxygen concentration is present. In such water, oxygen levels are relatively high near the surface, and they diminish with depth.[4] Studies showed that Mn(III) was also present in the sea floor mud, not only at the Black Sea, but also at a Gulf of Saint Lawrence and a Delaware salt marsh having the same oxygen gradient.[4] Soluble Mn(III) is likely stabilized by organic or inorganic ligands, and the research team thinks that it accounts for up to 90% of dissolved manganese.[3] It's produced by oxidation of dissolved Mn(II) and by reductive dissolution of MnO2 by biological or non-biological processes.[3] As I wrote earlier, precipitation of metal hydroxides by microorganisms is a process in the formation of manganese nodules, and the published study offers more insight into this mechanism. Says study coauthor, Andrew Madison, who did this research as part of his graduate studies at the University of Delaware,
"In sediments, bacteria prefer to consume molecular oxygen and nitrate first due to their high energy gain... After those are consumed, bacteria then couple organic matter oxidation to manganese oxide reduction, which can produce soluble manganese(III)."[3]This research was funded by the National Science Foundation and the Natural Sciences and Engineering Research Council of Canada.[3]
References:
- Average annual nickel prices through 1990, US Geological Survey
- Average annual nickel prices, 1991-2012, US Geological Survey.
- Andrew S. Madison, Bradley M. Tebo, Alfonso Mucci Bjorn Sundby and George W. Luther III, "Abundant Porewater Mn(III) Is a Major Component of the Sedimentary Redox System," Science, vol. 341, no. 6148 (August 23, 2013), pp. 875-878.
- Teresa Messmore, "Morphing manganese - UD researchers report new discovery in 'Science' about manganese in aquatic environments," University of Delaware Press Release, August 22, 2013.
- Average annual nickel prices, 1991-2012, US Geological Survey.