
Nanomixing and Nanoparticle Thermodynamics
October 21, 2013 Nanoscale objects are interesting because they behave differently than larger objects. The physics and chemistry discovered for larger objects need to be modified for nanoparticles. Some reasons for the different behavior of nanoparticles are the larger ratio of surface atoms to interior atoms, and their small size compared with a wavelength of light. I mentioned some problems associated with mixing of powders in a previous article (Blending, March 11, 2008). Liquid mixing is usually easier to accomplish, but there are still problems, as oil and water mixing demonstrates. The usual solution to getting hydrophilic liquids (water) and lipophilic (oil) liquids to mix is by adding a surfactant. Surfactant molecules have a hydrophilic head and a lipophilic tail, so they intermediate between the two different liquids. A research team from the University of Washington (Seattle, Washington) and the Pacific Northwest National Laboratory (Richland, Washington) published a paper earlier this year describing their experiments on nanoscale mixing of liquids.[1-2] Their paper in the Proceedings of the National Academy of Sciences demonstrates another example that nanoscale materials behave differently than their macroscale counterparts.[1] In this case, the effect likely arises from the fact that a greater number of molecules are at the flow boundary than at the bulk. The team used the device shown schematically in the figure. Mixing is done by passing liquids through posts with a micrometer-sized gap. As the liquid flows through the device it deforms quickly.[2] Their test liquids were mixtures of water, cetyltrimethylammonium bromide, which is weakly viscoelastic, sodium salicylate, which is strongly viscoelastic and shear-thinning, and a surfactant.[1]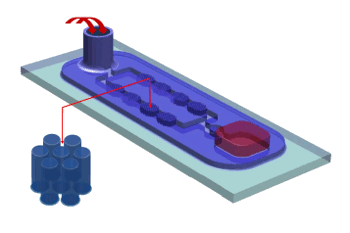 | Schematic diagram of the nanofluidic mixer. Liquids are injected at the back and forced through the posts for mixing. (University of Washington image.)[2] |
 | This electron micrograph shows the structure of a liquid with a gel-like consistency generated by the nanomixer in the previous figure. (image: Environmental Molecular Sciences Laboratory of the University of Washington.)[2] |
in which B is the spectral radiance, T is the absolute temperature, kB is the Boltzmann constant, h is the Planck constant, and c is the speed of light of the surrounding medium. Plotting this function gives you the familiar blackbody curve. Max Planck developed this law in 1900 as the first expression of quantum mechanics, and it applies generally to a wide variety of objects from stars to charcoal briquettes, whether hot or at room temperature. Planck himself realized that the law would not apply to very small objects, since an object cannot efficiently radiate thermal energy at wavelengths larger than its dimension.[5] An example from electrical engineering would be the inefficient transmission and reception of radio waves by a short antenna. The Vienna University of Technology team developed a generalized radiation formula from first principles that applies at the nanoscale to arbitrarily-shaped objects.[4] They tested their formula using a silica fiber with a diameter of 500 nanometer, which is smaller than a thermal wavelength.[4] They were able to measure the fiber temperature very accurately by measuring its optical path length, and they were able to relate this to the optical energy.[5]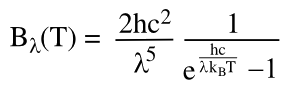
 | A picture, even an artist's conception, is worth a thousand words. A thinned optical fiber as used in the study. (Vienna University of Technology image.) |
"We could show that the fibers take much longer to reach their equilibrium temperature than a simple application of Planck’s law would suggest... However, our findings are in perfect agreement with the more general theory of fluctuational electrodynamics, which allows one to take the geometry and the size of the body into account."[5]This research will be useful in calculations involving the affect of atmospheric aerosols on climate, since soot particles are nanoscale.[5]
References:
- Joshua J. Cardiel, Alice C. Dohnalkova, Neville Dubash, Ya Zhao, Perry Cheung and Amy Q. Shen, "Microstructure and rheology of a flow-induced structured phase in wormlike micellar solutions," Proc. Natl. Acad. Sci., vol. 110, no. 18 (April 30, 2013), pp. E1653-E1660 .
- Michelle Ma, "New device could cut costs on household products, pharmaceuticals," Washington University Press Release, April 12, 2013.
- Rainbow of Nanoparticle Colors is 'Hot' Development from Xu Research Group , Old Dominion University Press Release, October 7, 2010.
- C. Wuttke and A. Rauschenbeutel, "Thermalization via Heat Radiation of an Individual Object Thinner than the Thermal Wavelength," Phys. Rev. Lett. vol. 111, no. 2 (July 12, 2013), Document No. 024301 (2013) [5 pages].
- Florian Aigner, "Heat Radiation of Small Objects: Beyond Planck's Equations," Vienna University of Technology Press Release No. 60/2013, July 8, 2013.
- Michelle Ma, "New device could cut costs on household products, pharmaceuticals," Washington University Press Release, April 12, 2013.