
Copper Nanowires for Solar Cells
December 6, 2013 You need electrical conductors to make electrical devices. You can use copper wire for larger devices, like table lamps and motors, but integrated circuits need to have small conductive traces of metal applied directly to the semiconductor chip. Although aluminum, and now copper, are used as conductors on integrated circuits, they aren't the only high conductivity (low resistivity) metals, as the following table shows.| Electrical Conductors (Data at 20°C from Wikipedia) (ρ is the resistivity, the reciprocal of conductivity.) |
| Material | ρ (10-8Ω-m) | Material | ρ (10-8Ω-m) | |
| Silver | 1.59 | Iron | 10 | |
| Copper | 1.68 | Platinum | 10.6 | |
| Gold | 2.44 | Tin | 10.9 | |
| Aluminum | 2.82 | 1010 Carbon steel | 14.3 | |
| Calcium | 3.36 | Lead | 22 | |
| Tungsten | 5.60 | Titanium | 42 | |
| Zinc | 5.90 | Stainless Steel † | 69 | |
| Nickel | 6.99 | Mercury | 98 | |
| Lithium | 9.28 | Nichrome | 110 |
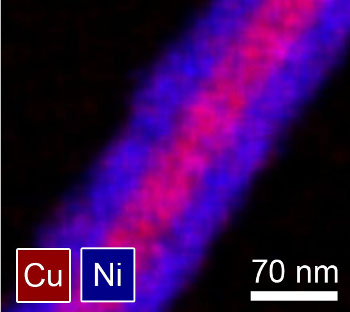 | Image of a nickel-coated copper nanowire. (Image supplied by Benjamin Wiley, used with permission). |
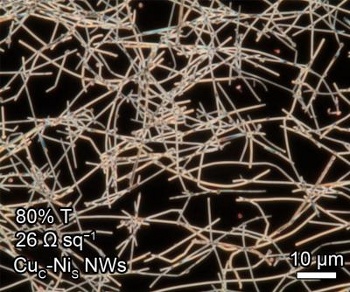 | Dark-field optical microscopy of a network of copper nanowires. (Duke University image by Zuofeng Chen.) |
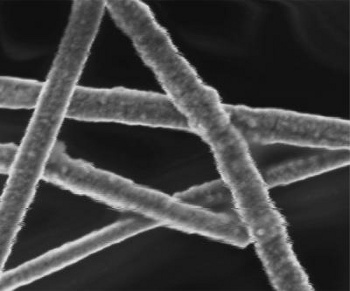 | Scanning electron micrograph of nickel-coated copper nanowires intended for water photolysis. (Duke University image by Zuofeng Chen.) |
References:
- US Geological Survey, Indium - USGS Mineral Resources Program, Mineral Commodity Summaries, 2013 (201 page PDF file).
- Jennifer Marcus, "UCLA team develops highly efficient method for creating flexible, transparent electrodes," UCLA Press Release, November 21, 2011.
- Rui Zhu, Choong-Heui Chung, Kitty C. Cha, Wenbing Yang, Yue Bing Zheng, Huanping Zhou, Tze-Bin Song, Chun-Chao Chen, Paul S. Weiss, Gang Li and Yang Yang, "Fused Silver Nanowires with Metal Oxide Nanoparticles and Organic Polymers for Highly Transparent Conductors," ACS Nano, vol. 5. no. 12 (October 28, 2011), pp. 9877-9882.
- Ashley Yeager, "Copper-Nickel Nanowires Could Be Perfect Fit For Printable Electronics," Duke University Press Release, May 29, 2012.
- Aaron R Rathmell, Minh Nguyen, Miaofang Chi and Benjamin John Wiley, "Synthesis of Oxidation-Resistant Cupronickel Nanowires for Transparent Conducting Nanowire Networks," Nano Letters, vol. 12, no. 6 (May 29, 2012), pp. 3193-3199.
- Zuofeng Chen, Aaron R. Rathmell, Shengrong Ye, Adria R. Wilson and Benjamin J. Wiley, "Optically Transparent Water Oxidation Catalysts Based on Copper Nanowires," Angew. Chem. Int. Ed., Online before Print (October 18, 2013), DOI: 10.1002/anie.201306585.
- Copper Promises Cheaper, Sturdier Fuel Cells, Duke University Press Release, November 22, 2013.
- Jennifer Marcus, "UCLA team develops highly efficient method for creating flexible, transparent electrodes," UCLA Press Release, November 21, 2011.