
Seawater Uranium
August 24, 2012 About two years ago, I published an article (Seawater Uranium, July 15, 2010) about the possibility of economically extracting uranium from seawater. As the world's carbon footprint increases at an alarming rate, it appears that nuclear energy might be the most environmentally friendly energy source we have. The main caveat here is that we need to prevent nuclear accidents, which have been far too many; and, we need a way to safely store radioactive waste. There is still innovation happening in nuclear technology, some of which you might have missed if "Bill Gates" is not a key phrase that you track. Bill Gates, who invested $35 million in TerraPower, the nuclear power start-up investigating traveling wave reactors, was in the news recently for another nuclear initiative. He's working with South Korea on development of a sodium-cooled fast reactor. Such reactors can use the spent fuel rods from conventional nuclear reactors, thereby eliminating much of our waste problems.[1] Conventional nuclear reactors operate as pressurized water reactors, boiling water reactors, or supercritical water reactors. Conventional nuclear reactors need to use enriched uranium as fuel, and this fuel is consumed over the course of a few years, leading to our current radioactive waste storage crisis. Traveling wave reactors can use depleted uranium as a fuel, once they are started, and no further fuel is required for many decades. All these reactors require uranium as a fuel, and this is mined just like other minerals. Unfortunately, estimates of available mined uranium show that there's only enough for about a century's operation of the present reactor base.[2] Four billion metric tons of uranium are estimated to be dissolved in seawater,[3] which is hundreds of times the quantity as mine reserves. However, the oceans have a lot of water, so the uranium concentration is just three parts per billion. Extraction by strictly chemical means is not possible. The uranium is dissolved with many other metals, and the total salt concentration is about 3.5%. Before Fukushima, the Japanese were especially concerned with maintaining their supply of uranium for nuclear power. At that time, about thirty percent of Japan's electrical power came from nuclear reactors. In 2003, Japanese scientists developed chemically-coated plastic fibers that were woven into a mat and placed into the sea. The test mat harvested a kilogram of uranium from seawater that was extracted by an acid rinse.[4] In continuing work, the Japanese Atomic Energy Commission developed a synthetic polymer fiber that adsorbs uranium. A thirty day exposure of fibers of this polymer to flowing seawater in 2010 resulted in a yield of 1.5 grams of yellowcake uranium per kilogram of fiber.[3] This idea of trapping uranium in treated fibers is being pursued by many others, and their research was recently presented at a symposium at the 244th National Meeting & Exposition of the American Chemical Society, August 19-23, 2012, Philadelphia, Pennsylvania. Abstracts of all twenty-five symposium presentations can be found at reference 5.[5] Fundamental studies are always welcome, and the symposium included the presentation, "Influence Of Temperature On Uranium Adsorption From Seawater," by Jungseung Kim of Oak Ridge National Laboratory.[5] I list a few of the other titles, below:• Electrospun Chitin Nanofibers For Uranyl Absorbant Materials, Chris S. Griggs, The University of Alabama.The list above includes an anticipated mix of material choices, but one material stands out. Chitin? Chitin is a polymeric protein found in the exoskeletons of crab, lobster, shrimp, and insects. It's a tough material, it can be made into very fine fibers by electrospinning, so it's a useful substrate for uranium extraction.[4]
• Extraction Of Uranium With Regenerated Chitin From The Dissolution Of Shrimp Shells In Ionic Liquid, Robin D. Rogers, The University of Alabama.
• Amidoxime-Grafted Mesoporous Carbon And Porous Organic Gel Sorbents For Extraction Of Uranium From Seawater, Suree Brown, University of Tennessee.
• Isolating Trace Seawater Uranium With Polymer Functionalized Porous Carbon, Yanfeng Yue, Oak Ridge National Laboratory.
• Funcitonalized Carbon Materials As Uranium Adsorbents, Richard Mayes, Oak Ridge National Laboratory.
• Synthesis, Development, And Testing Of Uranium Adsorbent Materials, Yatsandra Oyola, Oak Ridge National Laboratory.
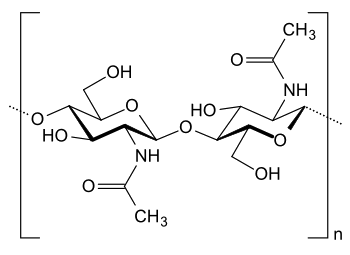 | Structural unit of chitin. (Structural diagram by D. Schanz, via Wikimedia Commons). |
 |
| Monthly uranium spot prices, via Via Wikimedia Commons. |
References:
- Bill Gates to Develop New Nuclear Reactor with Korea, englishnews@chosun.com, August 20, 2012.
- Uranium resources sufficient to meet projected nuclear energy requirements long into the future, Nuclear Energy Agency Press release, June 3, 2008.
- Sally Adee and Anne-Marie Corley, "Uranium From Seawater," IEEE Spectrum Online, June, 2010.
- Uranium from seawater idea boosted with shrimp shells, BBC, August 22, 2012.
- Advances in decades-old dream of mining seawater for uranium, American Chemical Society Press Release, August 21, 2012.
- ORNL technology moves scientists closer to extracting uranium from seawater, Oak Ridge National Laboratory Press Release, August 21, 2012.
- Advances in decades-old dream of mining seawater for uranium, American Chemical Society Press Release, August 21, 2012.
- Uranium resources sufficient to meet projected nuclear energy requirements long into the future, Nuclear Energy Agency Press release, June 3, 2008.