
Tin Pest and Purple Plague
April 24, 2012 The development of integrated circuitry was not without its surprises, and it offered new research opportunities for my fellow materials scientists. One problem involved the connection of the electrical signals from the silicon chips to the outside world. Nowadays, copper is used as the conductor in high density integrated circuits to route voltage and current between transistors and other circuit elements. This copper Damascene process is replacing the earlier technology in which aluminum was used for these on-chip connections. The connection to the package terminals is done with thin, highly conductive and very flexible gold wires. Bonding of the gold wires to the aluminum can be done by pressing a heated gold wire on the aluminum bonding pad with a little rubbing provided by an ultrasonic excitation of the bonding ferrule. Pull testing will prove that the connection is mechanically strong, as will proper electrical conductance of the joint. The problem in the gold-aluminum bond happens when too much heat is used to make the connection. In those cases, you end up with a low conductance joint characterized by either a white or purple coloration. These problems were poetically named the white and purple plagues. An illustration of a purple plague connection of gold to aluminum is shown in the figure.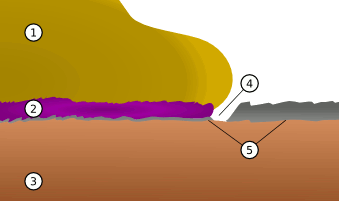 | A purple plague join (2) between gold (1) and aluminum (5) on a silicon chip (3). Growth of the AuAl2 intermetallic can cause a void in the aluminum (4). (Via Wikimedia Commons). |
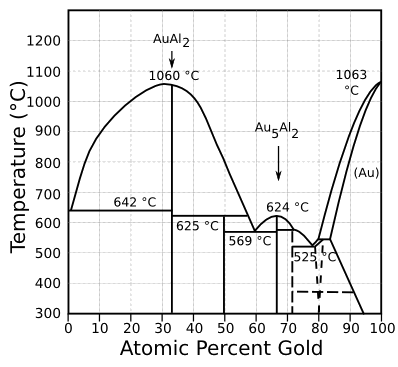 | The gold-aluminum phase diagram. The intermetallic compounds, AuAl2 (purple plague) and Au5Al2 (white plague) are shown. (Modified Wikimedia Commons image). |
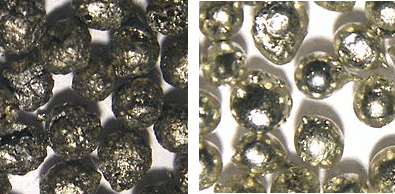 | Alpha (left) and beta (right) forms of tin. (Via Wikimedia Commons). |
References:
- Michael Barthelmy, "Problems With Pure Tin Coatings," NASA, January 17, 2001.
- Kenneth A. LaBel and Michael J. Sampson, "Developing a NASA Lead-free Policy for Electronics - Lessons Learned," TRISMAC 2008 Conference, April 15, 2008.
- R. Pfister and P. Pugnat, "Tin Pest: A Forgotten Issue in the Field of Applied Superconductivity?" arXiv Preprint Server, April 6, 2012.
- The Wizard of Oz, 1939, Victor Fleming, George Cukor, Mervyn LeRoy, Norman Taurog and King Vidor, Directors, Internet Movie Database.
- Kenneth A. LaBel and Michael J. Sampson, "Developing a NASA Lead-free Policy for Electronics - Lessons Learned," TRISMAC 2008 Conference, April 15, 2008.
