
Flow Batteries
July 18, 2012 An electrochemical battery has its energy stored in the potential energy of the chemical reactions it contains. In a lead-acid battery, the net discharge reaction isPb + PbO2 + 2H2SO4 --> 2PbSO4 + 2H2OThe reaction has an enthalpy (ΔH°) of -549 kJ, per starting mole of lead (Pb); the negative sign indicating a reaction in which we get some energy. Lead and lead dioxide (PbO2) are somewhat heavy, so it takes quite a bit of mass to get this energy. There is, however, a lot of energy to be had. Since a watt is a joule per second, 549 kJ is about 150 watt-hour. In theory, a ten pound battery could run a hair dryer for about half an hour. The volumetric energy density is constrained by the volume of the sulfuric acid (H2SO4), which is in a water solution. Right away, we take a volume penalty, since the 4.2 Molar sulfuric acid used is just a third H2SO4, and two-thirds water. Although lead-acid cells are rechargeable, once they're charged, that's the limit of your energy storage. Any extra energy you have can't be stored, so it's lost. Instead of having huge electrochemical batteries, wouldn't it be nice if we could have a smaller device that converts the energy into a liquid without consuming the electrodes in the process? We could then pump, or pour, this energy into suitable storage vessels, and then put this liquid energy back into the cell when we need to extract some power. This is reminiscent of the energon cubes in the Transformers animated television series. This science fiction is actually fact in a device called a flow battery; specifically, a redox flow battery. All the chemical reactions are contained in the solution, and the electrode materials are not consumed. The power you can get from such a flow battery depends in the electrode area, so our battery still needs some heft. However, the fluid volume depends only on the capacity of our storage tanks; and, the cost of a tank becomes less per unit volume as the tank volume increases. Flow batteries are a possible solution to load balancing in renewable energy systems, such as wind and solar energy. Such energy sources function intermittently, at the whim of nature, so you need to store energy while the sun shines, so to speak, and use it at a later time. Flow batteries eliminate the need to have a multitude of cells, formed from expensive materials, in a parallel combination. A team of scientists from Drexel University's A.J. Drexel Nanotechnology Institute has applied the flow battery concept to supercapacitors. In this case, the electrolyte is replaced by a slurry of carbon particles and electrolyte. Just as in standard supercapacitors, the energy is stored in an electric double layer at the charged carbon particles.[1-2]
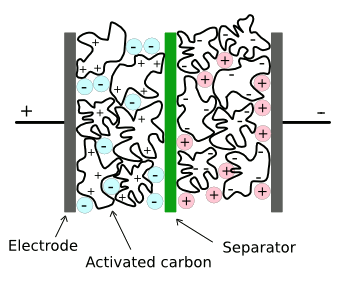 | Structure of a conventional supercapacitor. There are separate ionic liquids that are negatively and positively charged. One disadvantage of current materials is that these ionic liquids decompose at voltages above a few volts. (Via Wikimedia Commons, modified). |
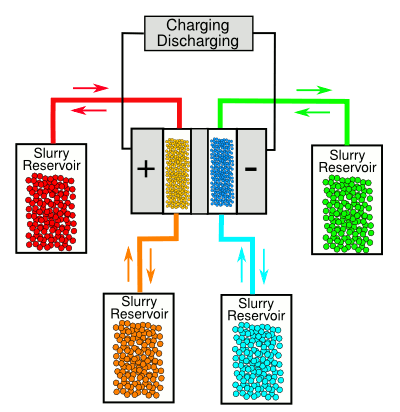 | The Drexel University flow battery. (Diagram rendered by the author using Inkscape). |
“We have observed very promising performance so far, being close to that of conventional packaged supercapacitor cells... However, we will need to increase the energy density per unit of slurry volume by an order of magnitude, and achieve it using very inexpensive carbon and salt solutions to make the technology practical."[2]
References:
- Volker Presser, Christopher R. Dennison, Jonathan Campos, Kevin W. Knehr, Emin C. Kumbur and Yury Gogotsi, "Electrochemical Flow Cells: The Electrochemical Flow Capacitor: A New Concept for Rapid Energy Storage and Recovery," Advanced Energy Materials, vol. 2, no. 7 (July, 2012), pp. 895-902.
- Britt Faulstick, "Making "Renewable" Viable: Drexel Engineers Develop New Technology for Grid-Level Electrical Energy Storage," Drexel University Press Release, July 10, 2012.
- Supplemental videos for ref. 1, Advanced Energy Materials, vol. 2, no. 7 (July, 2012).
- Britt Faulstick, "Making "Renewable" Viable: Drexel Engineers Develop New Technology for Grid-Level Electrical Energy Storage," Drexel University Press Release, July 10, 2012.