
Environmental Mercury
January 9, 2012 When I was younger, the environmental consequences of mercury pollution were quite apparent in the many photographs of victims of severe mercury poisoning in Minamata, Japan. If we look past the immediate cause of this human tragedy, we see that the root cause is the industrial utility of mercury, a material that's been used by humans for thousands of years. Mercury is unique in being a metal that's liquid at room temperature. It's melting point is quite a bit lower than that, -38.83 °C (-37.89 °F). Its low melting point, combined with its high thermal expansion coefficient (60.4 ppm/°C at 25 °C) makes it useful as a thermometer material. Gallium, which is somewhat distant from mercury in the periodic table, is also a liquid metal, but only in very hot rooms (melting point, 29.76 °C, 85.58 °F). Mercury is documented to have been refined more than two thousand years ago. About 2,250 years ago, Theophrastus (c. 371 - c. 287 BC) described cinnabar (HgS), the most common mercury-bearing ore, in his treatise, "On Stones." Although the common method of preparing mercury from cinnabar is by heating, Theophrastus described a mechanochemical method to obtain mercury; viz.,"It is made when cinnabar mixed with vinegar is ground in a copper vessel with a pestle made of copper."[1]This is the first published mechanochemical reaction, and the first published process for the preparation of a pure metal from a chemical compound.[2]
 |
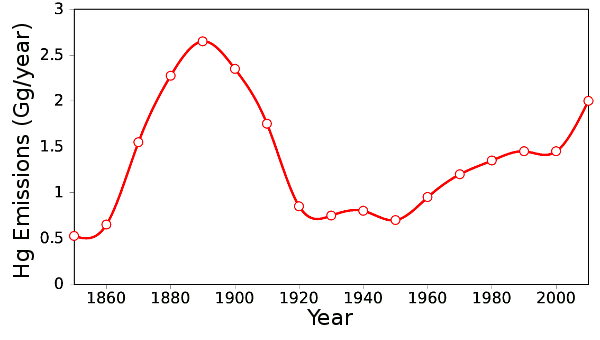 Graph via Gnumeric, using data from Ref. 4. [4]
Now that Asia is going through its initial period of industrial development, Asia has surpassed Europe and the Americas as an environmental mercury source.[3] Mercury in the air is one thing, but some batteries contain mercury as well, and these consumer products are just as likely to be discarded into the usual household waste streams as to be recycled.[3]
The study authors state that Earth's environmental load of mercury will take two thousand years to be cleansed from the environment by combining with crustal materials.[3] This research was supported by the US Department of Energy, the National Science Foundation and the Harvard University NIEHS Center for Environmental Health.[3]
Graph via Gnumeric, using data from Ref. 4. [4]
Now that Asia is going through its initial period of industrial development, Asia has surpassed Europe and the Americas as an environmental mercury source.[3] Mercury in the air is one thing, but some batteries contain mercury as well, and these consumer products are just as likely to be discarded into the usual household waste streams as to be recycled.[3]
The study authors state that Earth's environmental load of mercury will take two thousand years to be cleansed from the environment by combining with crustal materials.[3] This research was supported by the US Department of Energy, the National Science Foundation and the Harvard University NIEHS Center for Environmental Health.[3]
 | Mercury was often used in toy mazes. A small droplet of mercury could be directed through a channel from a starting point to a finish point.[5] (Image: Department of Ecology, State of Washington). |
References:
- Earle Radcliffe Caley and John F. C. Richards, Eds., "Theophrastus on Stones: Introduction, Greek Text, English Translation, and Commentary," Ohio State University Press (Columbus, 1956). Greek text available, here.
"There is also a natural and a prepared kind of cinnabar. The cinnabar in Iberia, which is very hard and stony, is natural, and so is the kind found in Colchis. They say that this is found on cliffs and is brought down by arrows that are shot at it. The prepared kind comes from one place only, a little above Ephesos."
- Laszo Takacs, "Quicksilver from cinnabar: The first documented mechanochemical reaction?" JOM Journal of the Minerals, Metals and Materials Society, vol. 52, no. 1 (January 9, 2000), pp. 12-13.
- Mercury releases into the atmosphere from ancient to modern times, ACS News Service Weekly PressPac, December 14, 2011.
- David G. Streets, Molly K. Devane, Zifeng Lu, Tami C. Bond, Elsie M. Sunderland and Daniel J. Jacob, "All-Time Releases of Mercury to the Atmosphere from Human Activities," Environ. Sci. Technol., vol. 45, no. 24 (November 9, 2011), pp 10485-10491.
- Mercury in Toys and Novelties, Department of Ecology, State of Washington.
- Laszo Takacs, "Quicksilver from cinnabar: The first documented mechanochemical reaction?" JOM Journal of the Minerals, Metals and Materials Society, vol. 52, no. 1 (January 9, 2000), pp. 12-13.