
Carbon Voltaic Energy
June 27, 2012 We live in a world of constant flow, much of which is useful. Hydroelectricity is actually a form of solar energy. It's created from the flow of water, and it presently provides a little more than six percent of the electricity in the United States. The flow of ocean water in the tides is another source of electrical energy, as I described in a recent article (Tidal Forces, June 20, 2012). You could claim a small fraction of that as being solar energy, but that flow is mostly the work of the Moon. Flow on smaller scales yields electrical power in some unusual ways. Rubbing certain materials together generates static electricity by the triboelectric effect, and this trick will work also with fluid flow. In one demonstration, a voltage of 300 millivolts (mV) was obtained with a flow of pure water at 45 cm3/sec in a millimeter diameter pipe.[1] This is also a cautionary tale of what materials you can use in gasoline filling pipes. Carbon nanotubes will generate a few millivolts when water flows over them because of coupling between ions in the water and charge carriers in the nanotubes. Since graphene is the wonder material of the new millennium, it's only reasonable that scientists would try it in similar triboelectric experiments. Engineers at Rensselaer Polytechnic Institute (Troy, New York) and Rice University (Houston, Texas) found that adding ions, in the form of hydrochloric acid (HCl), to the water gives an order of magnitude higher voltage when the flow is over a graphene film (see figure).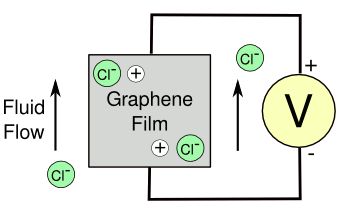 | Flow of chlorine ions generating a current in a graphene film. The moving chlorine ions might drag associated electron holes in the graphene sheet. (Drawing rendered by the author using Inkscape). |
 | When your funding is good, you reach for the Kapton tape (right), but most of the time, it's the duct tape (left). (Photo by author, via Wikimedia Commons). |
 | Graphite thermal energy device built from pencils. The green color of the solution indicates that it's a nickel(II) chloride solution. (Via arXiv Preprint Server).[4] |
References:
- B. Raveloa, F. Duvala, S. Kanea and B. Nsomb, "Demonstration of the triboelectricity effect by the flow of liquid water in the insulating pipe," Journal of Electrostatics, vol. 69, no. 6 (December, 2011), pp. 473-478.
- Prashant Dhiman, Fazel Yavari, Xi Mi, Hemtej Gullapalli, Yunfeng Shi, Pulickel M. Ajayan and Nikhil Koratkar, "Harvesting Energy from Water Flow over Graphene," Nano Letters, vol. 11, no. 8 (August 10, 2011), pp. 3123-3127.
- Richard Reeves, "A Force of Nature: The Frontier Genius of Ernest Rutherford" (W. W. Norton, December 3, 2007, ISBN-13:978-0393057508), via Amazon.
- Zihan Xu, and Guo'an Tai, "Electricity generated from Ambient Heat by Pencils," arXiv Preprint Server, June 17, 2012.
- Zihan Xu, Guoan Tai, Yungang Zhou, Fei Gao, Kin Hung Wong, "Self-Charged Graphene Battery Harvests Electricity from Thermal Energy of the Environment," arXiv Preprint Server, March 12, 2012.
- Edwin Cartlidge, "Sparks fly over graphene energy device," Nature Blog, March 15, 2012.
- Since I'm a thermodynamicist, one experiment I would do is light an external LED while monitoring the device temperature in an insulated vessel, such as a Dewar flask. The device should cool, since conservation of energy is always supposed.
- Prashant Dhiman, Fazel Yavari, Xi Mi, Hemtej Gullapalli, Yunfeng Shi, Pulickel M. Ajayan and Nikhil Koratkar, "Harvesting Energy from Water Flow over Graphene," Nano Letters, vol. 11, no. 8 (August 10, 2011), pp. 3123-3127.