
Vegard and the Periodic Table
January 24, 2012 All materials scientists are familiar with Vegard's law. This law simply states that when you combine elements to form an alloy, the lattice constant will follow a linear trend with the element concentrations, provided that there is no phase change.[1] I relied on Vegard's law often when I needed to match epitaxial layers to a particular substrate crystal. A recent example of Vegard's law appears in the figure.[2] Vegard is featured in a recent paper by Helge Kragh, a professor in the Department of Physics and Astronomy, Aarhus University (Aarhus, Denmark).[3]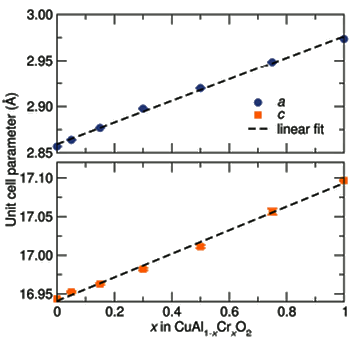 | Vegard's law as demonstrated in the lattice constant increase when chromium is substituted for aluminum in CuAlO2. CuAlO2 is a rhombohedral crystal, so there are two lattice constants that specify its unit cell. (Fig. 2 of Ref. 2, via the (arXiv Preprint Server). [2] |
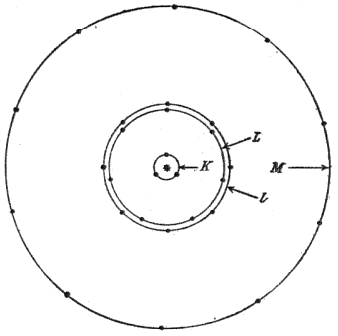 | Vegard's atomic model. This simple model explained the observed atomic spectra and the chemical properties of the elements. (Fig. 3 of Ref. 3, via the (arXiv Preprint Server). [3] |
References:
- Materialia Indica Classics in materials science: Vegard's law of linear relationship between lattice parameter and alloy composition, March 14, 2009 by "Guru".
- Phillip T. Barton, Ram Seshadri, Andrea Knöller and Matthew J. Rosseinsky, "Structural and magnetic characterization of the complete delafossite solid solution (CuAlO2){1-x}(CuCrO2){x}," arXiv Preprint Server, October 5, 2011.
- Helge Kragh, "An early explanation of the periodic table: Lars Vegard and X-ray spectroscopy," arXiv Preprint Server, December 16, 2011.
- Helge Kragh and Kristian Hvidtfelt Nielsen, "Spreading the gospel: The Bohr atom popularised," arXiv Preprint Server, December 12, 2011.
- Professor Lars Vegard (3/2 1880 – 21/12 1963), The Auroral Researcher, Physics Department, National University of Ireland Web Site.
- Phillip T. Barton, Ram Seshadri, Andrea Knöller and Matthew J. Rosseinsky, "Structural and magnetic characterization of the complete delafossite solid solution (CuAlO2){1-x}(CuCrO2){x}," arXiv Preprint Server, October 5, 2011.