
Edison's Nickel-Iron Battery Modernized
July 9, 2012 Electrochemistry shows that there are many possible battery systems. All you need are a reasonable pair of half-reactions, and a suitable electrolyte. Science is one thing, but technology is another. There are qualities required for a viable storage battery (rechargeable battery, or secondary cell), such as charging rate and the number of recharges allowed. The charging rate depends on such factors as the surface area of the electrodes and the mobility of ions in the electrolyte. The number of charging/discharging cycles allowed depends on the ability of the electrodes to hold themselves together as they transform from one chemical compound to another. For the lead-acid battery commonly used in conventional, internal combustion automobiles, this involves transformations of lead dioxide (PbO2) to lead sulfate (PbSO4), and lead to lead sulfate, in the presence of sulfuric acid. Since rechargeable batteries are an important part of mobile devices, there has been much research and development in battery systems that allow a high energy density along with a fast charging rate. The following figure shows the currently preeminent secondary battery systems and their energy densities in terms of both weight and volume.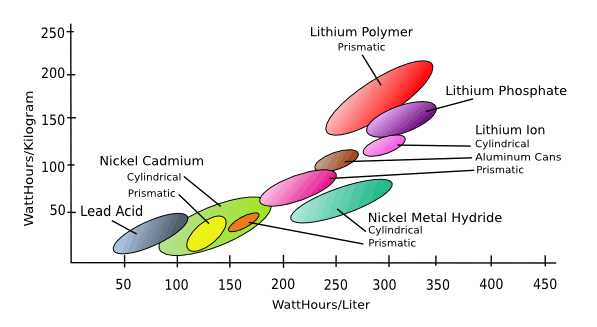 |
| Secondary cell energy density, in terms of both weight and volume. Research helps us to go farther to the upper right hand corner. (Via Wikimedia Commons). |
Cathode: 2NiOOH + 2H2O + 2e− <--> 2Ni(OH)2 + 2OH−This is not a very good storage battery by today's standard, since it has an energy density of just 50 Wh/kg, far towards the lower left quadrant of the figure. There's the further problem that nickel has become an expensive metal, costing about seven dollars a pound, as compared to the $0.80 per pound cost of lead. However, a century ago, when nickel wasn't too expensive and storage batteries were generally lead-acid, this battery type caught the interest of Thomas Edison. I wrote about Edison in a few previous articles ("His Master's Voice," August 15, 2011, "People Who Live in Concrete Houses...," June 30, 2011, and "Edison's Iron Mine," September 20, 2010). Edison's forte was not in invention, per se, but in improving and commercializing other's inventions. Edison didn't invent the incandescent light bulb, as commonly believed; instead, he perfected an inexpensive, long-lived version of it using a carbon filament derived from carbonized bamboo. The nickel–iron battery was invented by Swedish inventor, Waldemar Jungner, while conducting experiments on the nickel–cadmium battery he had invented in 1899. As most materials scientists would do, Jungner alloyed the cadmium with other metals, including iron, and showed that a nickel-iron rechargeable battery was possible. Edison took the idea to production in 1901 as a power source, superior to lead-acid, for electric automobiles.
Anode: Fe + 2OH− <--> Fe(OH)2 + 2e−
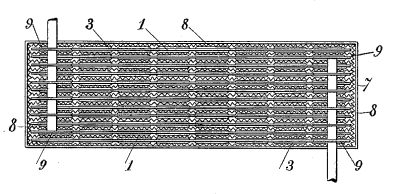 | Fig. 4 of US Patent No. 692,507, "Reversible Galvanic Battery," by Thomas Alva Edison, February 4, 1902. (Google Patents)[1]. |
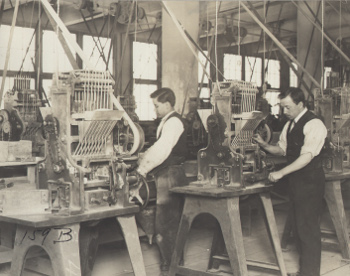 | Workers producing Edison storage batteries. Note how all machine power is derived from overhead drive shafts. (Edison Archive of the National Park Service). |
 | A woman using a washing machine powered by Edison storage batteries. (Edison Archive of the National Park Service). |
"Quoting power and energy density from small lab cells is not realistic... Real cells typically have capacities of only 20 percent of the numbers calculated in the lab."[4]
References:
- Thomas Alva Edison, "Reversible Galvanic Battery," US Patent No. 692,507, February 4, 1902
- A Bailey electric automobile, equipped with Edison storage batteries, and driven in a thousand mile endurance run in 1910.
- Mark Shwartz, "Stanford scientists develop ultrafast nickel-iron battery," Stanford University Press Release, June 26, 2012.
- Devin Powell, "Old battery gets a high-tech makeover," Science News, June 26, 2012.
- Hailiang Wang, Yongye Liang, Ming Gong, Yanguang Li, Wesley Chang, Tyler Mefford, Jigang Zhou, Jian Wang, Tom Regier, Fei Wei and Hongjie Dai, "An ultrafast nickel–iron battery from strongly coupled inorganic nanoparticle/nanocarbon hybrid materials," Nature Communications, vol. 3, Article No. 917, June 26, 2012 .
- A Bailey electric automobile, equipped with Edison storage batteries, and driven in a thousand mile endurance run in 1910.