
Pliable Resin
December 6, 2011 When I was still performing laboratory experiments, I would try to do these as cheaply as possible. There's no sense in spending too much money on a proof-of-concept device when that device might show that your concept is not that good. One of my favorite materials in this work was one of the many variants of "five-minute" epoxy. It's nice to have an adhesive that does its job after just a few moments of holding or clamping. Of course, there's the danger of doing things so fast and dirty that your device doesn't work simply because it's too shoddy. In the 1960s, when I was still in high school, a veteran electrical engineer told me about the circuit breadboards his colleagues would build that couldn't possibly work because of poor solder workmanship. Their circuit designs didn't fail, but their poor craftsmanship led them to believe that they did. | A Phrugal Physicist's Phriend. Five minute epoxy served the same purposes in my laboratory as sealing wax did in Rutherford's time. Photograph by Dzhang2680, (via Wikimedia Commons). |
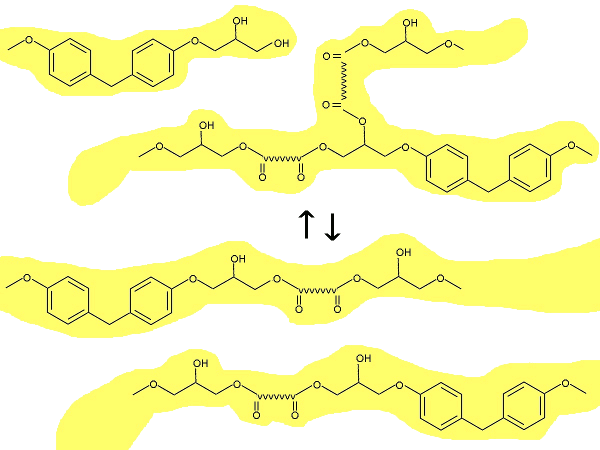 One example of a topological rearrangement process - transesterification in hydroxy-ester networks. See fig. 1B of Ref. 2. (Illustration by author).
This functionality derives from the material's ability to rearrange its molecules without changing the number of cross-links between molecular units. These materials can be designed to be either elastomeric, or hard and rigid, depending on composition. With application of heat, these materials can be shaped, as shown in the photograph, below. When used as the matrix phase in composites, these materials can compete with metals in some applications.[1]
One example of a topological rearrangement process - transesterification in hydroxy-ester networks. See fig. 1B of Ref. 2. (Illustration by author).
This functionality derives from the material's ability to rearrange its molecules without changing the number of cross-links between molecular units. These materials can be designed to be either elastomeric, or hard and rigid, depending on composition. With application of heat, these materials can be shaped, as shown in the photograph, below. When used as the matrix phase in composites, these materials can compete with metals in some applications.[1]
 | A strip of pliable resin being worked by hand over a hot air gun. Note the thermocouple arranged to allow fine control of the working temperature. (CNRS image). |
References:
- Priscilla Dacher, "New revolutionary material can be worked like glass," CNRS Press Release, November 16, 2011.
- Damien Montarnal, Mathieu Capelot, François Tournilhac and Ludwik Leibler, "Silica-Like Malleable Materials from Permanent Organic Networks," Science, vol. 334, no. 6058 (November 18, 2011), p. 965-968.
- Supporting Online Material for Ref. 2.
- This Week in Science: Editor summaries of this week's papers - Not So Thermoset, Science, vol. 334, no. 6058 (November 18, 2011), p. 875.
- Video of material pliability for Ref. 2.
- Damien Montarnal, Mathieu Capelot, François Tournilhac and Ludwik Leibler, "Silica-Like Malleable Materials from Permanent Organic Networks," Science, vol. 334, no. 6058 (November 18, 2011), p. 965-968.