
Phase Change Conductance
June 21, 2011 In a previous article (Nano Memory, September 30, 2010), I wrote about memory devices being developed from the reversible change in electrical conductance that some materials have when they change phase between amorphous and crystalline states. This effect was discovered by Stanford R. Ovshinsky, and it's often called The Ovonic effect.[1] This effect has become important as a memory technology known as phase-change memory. Materials that exhibit this effect are quite exotic, and also quite toxic. An important class of such materials is the chalcogenide glasses, specifically selenides and tellurides, such as Ge2Sb2Te5 (called GST). The amorphous state of these compounds has low conductivity, and the crystalline state has high conductivity. Application of proper voltages switches between these phases in a few tens of nanoseconds. This is remarkable, since these transitions involve an actual heating of the material. Such rapid transitions are possible, since the amount of material is very small, and it's in intimate contact with a heat-sinking substrate. There are other ways to change the conductance of a material, although not through a change in the intrinsic electrical properties of the material itself, but the conductance path through crystalline grains of the material, a process called percolation.[3-4] I wrote in two previous articles[5-6] how conductance in such materials can be changed by mechanical stress, an effect called piezoresistance.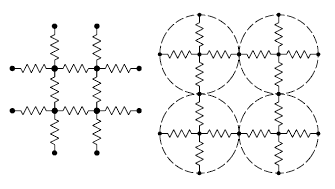 | One way to model electrical percolation in two-dimensions. Particles are modeled as resistive links to nearest neighbors. Fig. 1 of ref. 4, via arXiv |
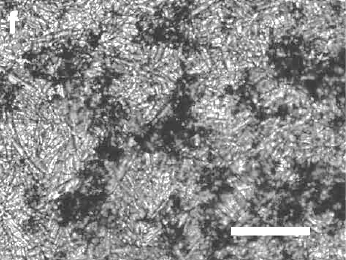 | Fig. 1(f) from ref. 7. An image of a network of graphite particles formed by freezing a graphite suspension in hexane. Graphite clusters are black, and the needle-like structure is the frozen hexane. The scale bar is 200 μm. |
References:
- Stanford R. Ovshinsky, "Reversible Electrical Switching Phenomena in Disordered Structures," Physical Review Letters, vol. 21, no. 20 (1968), pp. 1450-1453.
- David Lammers, "Resistive RAM Gains Ground," IEEE Spectrum (September 2010).
- A. S. Ioselevich and D. S. Lyubshin, "Percolation with excluded small clusters and Coulomb blockade in a granular system," arXiv Preprint Server, October 15, 2009.
- Yakov M. Strelniker, Shlomo Havlin, Richard Berkovits and Aviad Frydman, "Resistance distribution in the hopping percolation model," arXiv Preprint Server, June 7, 2005.
- This Blog, Piezomolecular Effect, February 22, 2011.
- This Blog, Carbon Nano-Aerogel, March 14, 2011.
- D.M. Gualtieri, "Aircraft icing sensor," U.S. Pat. No. 7,683,791, March 23, 2010.
- David L. Chandler, "New method found for controlling conductivity - Reversible control of electrical and thermal properties could find uses in storage systems," MIT Press Release, April 29, 2011.
- Ruiting Zheng, Jinwei Gao, Jianjian Wang and Gang Chen, "Reversible temperature regulation of electrical and thermal conductivity using liquid–solid phase transitions," Nature Communications, vol. 2, no. 4 (April 19, 2011), Article no. 289, pp. 1-6.
- David Lammers, "Resistive RAM Gains Ground," IEEE Spectrum (September 2010).