
Under Pressure
June 13, 2011 After my post-doc, I interviewed at the company for which I worked until my retirement, thirty years later. This company, at that time characterized as a "sleepy little chemical company," had decided to invest in research to reinvent itself with new product lines. To that end, it had hired some very prominent scientists in the early 1970s to staff a materials research center, and I was recommended there for an interview by a colleague of one of the managers. Part of the employment process was an interview with the director, Jack Gilman (December 22, 1925 - September 10, 2009). The company was engaged in research on the production of amorphous metals on an industrial scale, one of Jack's pet projects,[2] so we talked about those for a while. Jack was insistent that these metals had no order at all, whether long-range order or short-range order. I was likewise insistent that short-range order must exist, but perhaps it was just too hard to detect with the instrumentation he had at the time. I still got the job. My conjecture was proven about five years later, when short range order was discovered in metallic glasses. Atoms of metal will arrange themselves into orderly crystalline structures unless you try really hard to do otherwise. One interesting halfway-state between an ordered crystal and a completely amorphous solid is the quasicrystal, in which atoms have a regular arrangement when viewed locally, but their lattice doesn't have translational symmetry; that is, their lattice structure is not periodic. The first published example of a quasicrystal was an aluminum alloy with 14% manganese content.[1] Not surprisingly, this material was formed by rapid cooling, which presumably doesn't give the atoms sufficient time to arrange themselves into their most favored position; that is, a regular crystal. This discovery was significant, since the Al-Mn quasicrystalline phase persisted after heating six hours at 300°C. Heating one hour at 400°C did result in conversion to an Al6Mn crystal form. Rapid cooling is the key to making quasicrystals, and its also the key to making amorphous metals. The rapid cooling task is made easier in alloy compositions at "deep" eutectics; that is, eutectic compositions that solidify at a temperature much lower than their constituents. Heating, of course, will allow amorphous metals to revert to their preferred crystalline state. You can even perform a partial annealing to get small crystals in an amorphous matrix, something which allows important modification of a material's magnetic properties. As recent research shows, there's another, more complicated, way. Application of high pressure will likewise cause an amorphous-to-crystalline phase transition in a particular metallic glass; and by high pressure, I mean really high. It takes 250,000 bars of pressure (250,000 times atmospheric pressure at sea level), or 25 gigapascals.[3-5]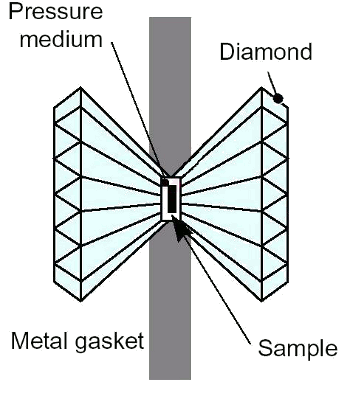 | Diamond anvil pressure cell. (Via Wikimedia Commons) |
References:
- D. Shechtman, I. Blech, D. Gratias and J. W. Cahn, "Metallic Phase with Long-Range Orientational Order and No Translational Symmetry," Physical Review Letters, vol. 53, no. 20 (November 12, 1984), p. 1951-1953. PDF available here.
- John J. Gilman, "Metallic Glasses," Science, vol. 208, no. 4446 (May 23, 1980), pp. 856-861.
- Qiaoshi Zeng, Hongwei Sheng, Yang Ding, Lin Wang, Wenge Yang, Jian-Zhong Jiang, Wendy L. Mao and Ho-Kwang Mao, "Long-Range Topological Order in Metallic Glass," Science, vol. 332, no. 6036 (June 17, 2011), pp. 1404-1406.
- Melinda Lee, "Metallic glass: A crystal at heart," DOE/SLAC National Accelerator Laboratory Press Release, June 16, 2011.
- Ho-kwang Mao, "Searching for the 'perfect glass'," Carnegie Institution Press Release, June 16, 2011.
- John J. Gilman, "Metallic Glasses," Science, vol. 208, no. 4446 (May 23, 1980), pp. 856-861.