
Lithium Ion Electrodes
December 13, 2011 Silicon, the miracle element of our present electronic age, is quickly being supplanted by its neighbor, carbon, in the periodic table of elements. One advantage that carbon has over silicon is that it exists in several alloptopic forms.| Portion of the periodic table of elements near carbon and silicon. (Via Wikimedia Commons). |
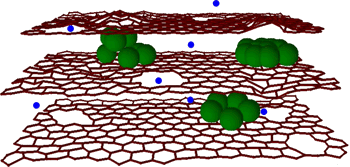 | A graphene-silicon composite anode for lithium ion batteries. (Via Northwest University). |
"We have found a way to extend a new lithium-ion battery's charge life by 10 times... Even after 150 charges, which would be one year or more of operation, the battery is still five times more effective than lithium-ion batteries on the market today."[7-8]One advantage that the graphene flakes have over graphite is that the lithium ions have a shorter travel time from the edge of the carbon to any region within. The defects in the graphene flakes, the 10-20 nm holes that allow a transverse flow of lithium through the flakes, are made by chemical oxidation.[7-8] Now that these lithium-ion electrodes, used as anodes in rechargeable batteries, have been optimized nearly as much as possible, it's time to concentrate on the cathode. Says Stanford's Yi Cui, who developed the silicon nanowire anode,[1]
"We are actually limited more by the cathode... Improving the anode will have a very big impact. But improving the cathode can have an even larger impact."[3]
References:
- Candace K. Chan, Hailin Peng, Gao Liu, Kevin McIlwrath, Xiao Feng Zhang, Robert A. Huggins and Yi Cui, "High-performance lithium battery anodes using silicon nanowires," Nature Nanotechnology, vol. 3, No. 1 (January, 2008), pp. 31-35.
- Hyunjung Kim, Byunghee Han, Jaebum Choo, Prof. and Jaephil Cho, Prof., "Three-Dimensional Porous Silicon Particles for Use in High-Performance Lithium Secondary Batteries," Angewandte Chemie, vol. 120, no. 52 (December 15, 2008), pp. 10305-10308.
- Peter Fairley, "Realizing Lithium-Battery Potential - Nanoporous silicon that soaks up ions without self-destructing can make better batteries," Technology Review, December 3, 2008.
- A. Magasinski, P. Dixon, B. Hertzberg, A. Kvit, J. Ayala and G. Yushin, "High-performance lithium-ion anodes using a hierarchical bottom-up approach," Nature Materials, vol. 9, no. 4 (April, 2010), pp. 353-358.
- Darren Quick, "Silicon-based anode to boost lithium-ion battery performance," Gizmag, April 8, 2010.
- Xin Zhao, Cary M. Hayner, Mayfair C. Kung and Harold H. Kung, "In-Plane Vacancy-Enabled High-Power Si–Graphene Composite Electrode for Lithium-Ion Batteries," Advanced Energy Materials, vol. 1, no. 6 (November, 2011), pp. 1079-1084.
- Megan Fellman and Sarah Ostman, "Better Batteries - New technology improves both energy capacity and charge rate in rechargeable batteries," Northwest University Press Release, November 14, 2011.
- Sarah Ostman, "Researchers Design Rechargeable Battery with Improved Charge Capacity, Rate," Northwest University McCormick School of Engineering and Applied Science Press Release, November 2, 2011.
- Hyunjung Kim, Byunghee Han, Jaebum Choo, Prof. and Jaephil Cho, Prof., "Three-Dimensional Porous Silicon Particles for Use in High-Performance Lithium Secondary Batteries," Angewandte Chemie, vol. 120, no. 52 (December 15, 2008), pp. 10305-10308.