
Salt Water Energy
May 20, 2011 In our quest for energy, we sometimes enter regions of unintended consequences. Three Mile Island, Chernobyl and Fukushima can be placed in this category, but we needn't stigmatize the nuclear industry, alone. A recent article in the Proceedings of the National Academy of Sciences reports how drilling for the natural gas found in shale has contaminated some groundwater supplies.[1] The noted environmentalist, Lester B. Lave of Carnegie Mellon University, who died on May 9, 2011,[2] founded the field of life-cycle assessment that analyzes the environmental impact of products from "cradle to grave." When we look at an automobile, we shouldn't focus just on the tailpipe emissions. We should look also at all the polluting processes that went into its manufacture, such as mining its metal and making the many sets of tires it will need over its lifetime. There's also the problem of its final disposition, perhaps in a landfill. The same type of environmental audit should be done for renewable energy devices. When you make your photovoltaics from cadmium or selenium, how does the decommissioning expense figure into your energy cost? More importantly, does the energy needed to manufacture your renewable energy device exceed the energy you'll get from it? There are also the costs involved in siting and installing your device, and its subsequent maintenance. The reason that most photovoltaics are on roofs is that land is generally too expensive to have a solar farm as its sole occupant. In 2009, Doriano Brogioli of the Università degli Studi di Milano-Bicocca, Monza, Italy, published the idea of an interesting energy generator in Physical Review Letters.[3] His device was a supercapacitor that's alternatively exposed to salt water and freshwater. Brogioli's effect is like that observed in a common physics experiment in which a capacitor is charged at a certain voltage, and then its dielectric is removed. When this happens, the voltage on the capacitor plates increases. The reason for this is that the voltage V on a capacitor is given as the ratio of its stored charge Q and its capacitance C; namelyV = Q/CSince the charge Q is stored on the conducting plates, the charge is unchanged when the dielectric is removed. Since the capacitance is decreased through removal of the dielectric, the voltage increases. More importantly, the energy stored in the capacitor increases. The stored energy of a capacitor U is given by
U = (1/2) C V2Since we're always mindful of conservation laws (in this case, physical, not environmental), where does this extra energy come from? It comes from the work we did in removing the dielectric. Our capacitor has become a transformer that changes mechanical work to electrical power.
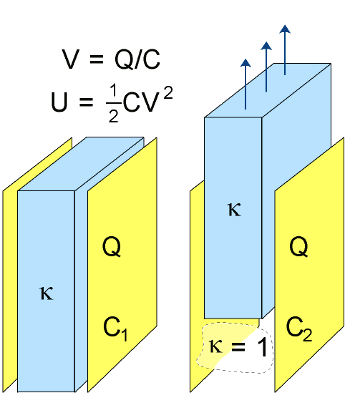 | Removal of a dielectric from a parallel plate capacitor. The charge remains constant, but the plate voltage and stored energy increase. Illustration by the author, via Wikimedia Commons |
ΔU = (1/2) (Q2/C) (κ - 1)Brogioli's device uses the double-layer capacitance effect found in supercapacitors. Large surface area carbon electrodes, like those used in supercapacitors, are placed in a saline solution and the resultant capacitor is charged. Just as in a supercapacitor, it's the ions in solution that are important. When voltage is applied to the salt water solution, the sodium and chlorine ions migrate to their appropriate electrodes. When fresh water is allowed to displace the saline, the capacitor energy increases as the ions diffuse away from the electrodes, increasing the plate voltage. Just as in the case of withdrawal of a solid dielectric from a parallel plate capacitor, work must be done to remove the ions at the electrodes. That's because the electrostatic forces try to hold the ions at the electrodes as the diffusion of fresh water carries them away. Brogioli's small test cell gave just five microjoules of energy per cycle, but he estimates a potential for 1.6 KJ per liter of fresh water.[3-4] One immediate problem is that the voltage must be kept below one volt so that chemical reactions don't take place. There is a similar problem in supercapacitors. Yi Cui, an Associate Professor of Materials Science and Engineering at Stanford University, and his research team have been extending Brogioli's design using a cathode of sodium-manganese dioxide (Na2−xMn5O10) nanorods that increase the surface area and allow a faster diffusion of the sodium ions in and out of the cathode.[5-7] Unfortunately, silver is needed as the anode. Using Pacific Ocean seawater and freshwater from Donner Lake, Cui's team obtained 74% percent of the possible energy that can be obtained from this mechanism. According to Cui, 50 cubic meters of freshwater per second would produce about 100 megawatts of power, which is enough electricity for about 100,000 households.[6] How much energy is available this way? A 1974 paper by Richard S. Norman[8] estimated that the freshwater runoff of the US alone yields about 10 gigawatts at 25% efficiency. Worldwide, it's as if each river running into the ocean were terminated in a 225 meter waterfall at its mouth. Of course, everything has its downside. As I wrote in a previous article (The Water Equivalent of Energy, June 1, 2010), freshwater is becoming a scarce material. This point is reiterated in a recent book review in Nature.[9] According to the World Bank, India's supplies of fresh water will be exhausted by 2050; and China, which has polluted about seventy percent of its water supplies, will run out of fresh water by 2030. Mixing freshwater with salt water might not be a good idea in the long run.
References:
- Stephen G. Osborn, Avner Vengosh, Nathaniel R. Warner andRobert B. Jackson, "Methane contamination of drinking water accompanying gas-well drilling and hydraulic fracturing," Proc. Natl. Acad. Sci., Published online before print, May 9, 2011, doi: 10.1073/pnas.1100682108.
- Dan Majors, "Obituary: Lester B. Lave / Carnegie Mellon economics professor and visionary researcher," Pittsburgh Post-Gazette, May 10, 2011.
- Doriano Brogioli, "Extracting Renewable Energy from a Salinity Difference Using a Capacitor," Phys. Rev. Lett. vol. 103, no. 5 (July 31, 2009).
- Lauren Schenkman, "Buzz Blog: Electricity From Salty Water," Physics Central, July 23, 2009.
- Fabio La Mantia, Mauro Pasta, Heather D. Deshazer, Bruce E. Logan and Yi Cui, "Batteries for Efficient Energy Extraction from a Water Salinity Difference," Nano Lett., vol. 11, no. 4 (March 17, 2011), pp 1810-1813.
- Louis Bergeron, "Stanford researchers use river water and salty ocean water to generate electricity," Stanford Report, March 28, 2011
- Dexter Johnson, "Nanomaterial Boosts Efficiency of Salinity Power Technology," IEEE Spectrum, May 06, 2011.
- Richard S. Norman, "Water Salination: A Source of Energy," Science, Vol. 186 no. 4161 (October 25, 1974), pp. 350-352.
- Margaret Catley-Carlson, "Environment: Water, water everywhere...," Nature, vol. 473, no. 7345 (May 5, 2011), pp.27-28.
- Dan Majors, "Obituary: Lester B. Lave / Carnegie Mellon economics professor and visionary researcher," Pittsburgh Post-Gazette, May 10, 2011.