
Water Photolysis by Antimony-Doped Gallium Nitride
September 1, 2011 I reviewed the photolysis of water (splitting water into hydrogen and oxygen using light energy) in two previous articles, Manganese Photolysis of Water (June 1, 2011) and Titania Photocatalysis (February 16, 2011). This would be one method of using solar energy to provide vehicular fuel. The other, of course, is photovoltaics for generating electricity. Research in water-splitting using light accelerated after the 1972 publication of an article in Nature by Akira Fujishima and Kenichi Honda.[1] They described a photoelectrochemical cell that used titania as a photoanode.[1-2] This cell was capable of water photolysis. One problem with the Fujishima-Honda photolysis cell is that it was inefficient in conversion of solar energy into hydrogen. Since titania, in its rutile crystal form, has a bandgap of 3.0 eV, it will only split water when illuminated by light shorter in wavelength than 413.75 nm. Less than 10% of solar energy appears in those wavelengths. Others have been researching ways to reduce the titania bandgap to increase efficiency.[3-4] I reviewed gallium nitride (GaN), an important semiconductor material for high speed and high power electronics, in a previous article (Gallium Nitride Crystals, July 29, 2010). Since gallium nitride in its zinc blende crystal structure has a bandgap of 3.2 eV, which is higher than titania, you wouldn't expect it to be a candidate as a photolysis material. Also, two-inch diameter single crystal wafers of GaN are priced at about a thousand dollars, although one company is working on a process to make less expensive GaN wafers. A recent paper in Physical Review B by a broadly-based research team with members at the University of Kentucky (Lexington, Kentucky), Daimler AG GR/PSS (Ulm, Germany), the Greek Foundation for Research and Technology, FORTH (Heraklio, Crete, Greece), the University of South Florida (Tampa, Florida) and the University of Louisville (Louisville, Kentucky) looks at the consequences of doping gallium nitride with antimony (Sb).[5-6] They found that a 2% doping of Sb in GaN will significantly reduce its band gap. Since GaN is a high temperature semiconductor, there's a possibly for efficient operation in solar concentrator arrays. Unfortunately, this is a computation and not an experiment, so I'll reserve judgment until I see the real thing.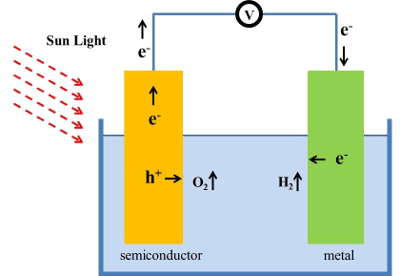 | Water photolysis using Sb-doped gallium nitride. (Image via University of Kentucky). |
References:
- Akira Fujishima and Kenichi Honda, "Electrochemical Photolysis of Water at a Semiconductor Electrode," Nature, vol. 238, no. 5358 (July 7, 1972), pp. 37-38.
- J. Keeney, D. H. Weinstein and G. M. Haas, "Electricity from photosensitisation of titanium," Nature, vol. 253, no. 5494 (February 27, 1975), pp. 719-720.
- Dan Krotz, "A Dash of Disorder Yields a Very Efficient Photocatalyst," Lawrence Berkeley Laboratory Press Release, January 28, 2011.
- Xiaobo Chen, Lei Liu, Peter Y. Yu and Samuel S. Mao, "Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals," Science, vol. 331, no. 6018 (February 11, 2011), pp.746-750.
- Keith Hautala, "Novel Alloy Could Produce Hydrogen Fuel from Sunlight," University of Kentucky Press Release, Aug 31, 2011.
- R. Michael Sheetz, Ernst Richter, Antonis N. Andriotis, Sergey Lisenkov, Chandrashekhar Pendyala, Mahendra K. Sunkara and Madhu Menon, "Visible-light absorption and large band-gap bowing of GaN1−xSbx from first principles," Phys. Rev. B, vol. 84, no. 7 (August, 15 2011), Document No. 075304 (4 pages).
- Gallium Statistics, U.S. Geological Survey, October 5, 2010
- J. Keeney, D. H. Weinstein and G. M. Haas, "Electricity from photosensitisation of titanium," Nature, vol. 253, no. 5494 (February 27, 1975), pp. 719-720.
