
Transparent and Conductive
June 10, 2011 There's a memorable scene in the fourth Star Trek Movie, "Star Trek IV: The Voyage Home" (a.k.a., "Kirk Saves the Whales"). Ship's engineer, Scotty, after a comical encounter with a computer mouse, inputs the structure of "transparent aluminum" into a CAD program. This fictional material has been identified with the actual materials, aluminium oxynitride and the sapphire crystalline form of aluminium oxide. Neither of these are pure aluminum; not only that, but they aren't electrical conductors like aluminum. Metals are good electrical conductors, but they are reflective, not transparent, since the electrons responsible for the conductivity also scatter photons. There are some metals that are both transparent and conductive, but only when they are very thin films. These are the "simple metals," copper, silver and gold. In my own experiments on thin silver films I was able to obtain somewhat transparent films with a modest conductivity, but nothing technologically significant. The semiconductor, gallium arsenide, is transparent in the infrared, and infrared microscopes are used to peer into gallium arsenide wafers for defects. If you want a material that's both transparent at visible wavelengths and electrically conductive, as for display screens and solar collectors, then indium-tin oxide (ITO) has been essentially the only developed candidate. I wrote about indium and the worldwide shortage of this element in a previous article (Indium, January 8, 2008). Indium, which has about the same abundance as silver (0.1 ppm), is a byproduct of zinc ore refining. The price of indium was as high as $1,000 per kilogram in 2005. In 2010, the estimated world consumption was 120 metric tons at an average price of about $560/kg.[1-2] Indium is combined with tin and oxygen with a typical composition of 90% In2O3 and 10% SnO2, by weight, to form indium-tin-oxide, a highly transparent and electrically conductive material. ITO reigns supreme, although there are similar materials, as follow:• Indium tin oxyfluoride - More difficult to etch, and it still contains indium.[3] • Zinc aluminum oxide - Not quite as transparent as ITO.[4] • Cadmium tin oxide - Highly toxic.[5] • Calcium aluminum oxide (Mayenite) - This material is the transparent insulating oxide 12CaO⋅7Al2O3 that's converted into an electrical conductor by thermal treatment in a hydrogen atmosphere and ultraviolet light exposure.[6]What's needed is a viable alternative to ITO that's inexpensive and easy to process. There are conducting polymers that are both transparent and conducting, such as poly-(3,4-ethylene dioxythiophene), also called PEDOT.[2] PEDOT is the leading candidate for use in flexible displays. Graphene layers have been proposed, also. Instead of the graphene form of carbon, carbon nanotube materials may also serve as an ITO replacement.
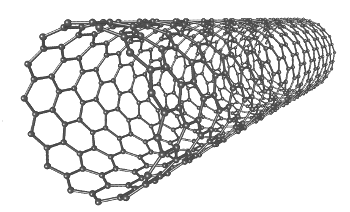 | A single-walled carbon nanotube. (Via Wikimedia Commons) |
References:
- US Geological Survey, Indium - USGS Mineral Resources Program, Mineral Commodity Summaries, 2011.
- Amy C. Tolcin, "Indium," U.S. Department of the Interior, U.S. Geological Survey, Mineral Commodity Summaries 2011, p. 74.
- S.-J. Jiang, Z.-C. Jin, and Claes G. Granqvist, "Low-refractive-index indium-tin-oxyfluoride thin films made by high-rate reactive dc magnetron sputtering," Appl. Opt. vol. 27, no. 14 (July 15, 1988), 2847-2850.
- W. Tanga and D.C. Camerona, "Aluminum-doped zinc oxide transparent conductors deposited by the sol-gel process," Thin Solid Films, vol. 238, no. 1 (January 15, 1994), pp. 83-87.
- Xiaonan Li, Timothy A. Gessert and Timothy Coutts, "The properties of cadmium tin oxide thin-film compounds prepared by linear combinatorial synthesis, Proceedings of the Second Japan-US Workshop on Combinatorial Materials Science and Technology, Applied Surface Science, vol. 223, no. 1-3, February 15, 2004, pp. 138-143.
- Katsuro Hayashi, Satoru Matsuishi, Toshio Kamiya, Masahiro Hirano and Hideo Hosono, "Light-induced conversion of an insulating refractory oxide into a persistent electronic conductor," Nature, vol. 419, no. 6906 (October 3, 2002), pp. 462-465.
- Ivo Jongsma, "Researchers find replacement for rare material indium tin oxide," Eindhoven University of Technology Press Release, April 11. 2011.
- Andriy V. Kyrylyuk, Marie Claire Hermant, Tanja Schilling, Bert Klumperman, Cor E. Koning and Paul van der Schoot, "Controlling electrical percolation in multicomponent carbon nanotube dispersions," Nature Nanotechnology (Published online April 10, 2011)
- Amy C. Tolcin, "Indium," U.S. Department of the Interior, U.S. Geological Survey, Mineral Commodity Summaries 2011, p. 74.