
Silver Nanowire Transparent Electrodes
December 19, 2011 Materials that are transparent at visible wavelengths and electrically conductive are important for display screens and photovoltaics. Indium-tin oxide (ITO, typical composition 90% In2O3 and 10% SnO2 by weight) is the most popular material for these applications, but indium is becoming scarce and expensive. I reviewed the worldwide shortage of indium in two previous articles, "Indium," January 8, 2008, and "Transparent and Conductive," June 10, 2011. In 2010, the estimated world consumption of indium was 120 metric tons at an average price of about $560/kg.[1-2] It's no wonder that materials scientists are researching ITO replacements. At this time, there are a few alternatives to ITO, although they all fall short in one way or another. The table below lists the prime contenders.| Material | Notable Properties |
| poly-(3,4-ethylene dioxythiophene) (PEDOT) | A conducting polymer suitable for flexible displays.[2] |
| Indium tin oxyfluoride | More difficult than ITO to etch, and it still contains indium.[3] |
| Zinc aluminum oxide | Not quite as transparent as ITO.[4] |
| Cadmium tin oxide | Highly toxic.[5] |
| Calcium aluminum oxide (Mayenite) | This material is the transparent insulating oxide 12CaO⋅7Al2O3 that's converted into an electrical conductor by thermal treatment in a hydrogen atmosphere and ultraviolet light exposure.[6] |
| Graphene and carbon nanotubes | Percolation networks of carbon nanotubes in polymers have about a hundred times less conductivity than ITO.[7-8] |
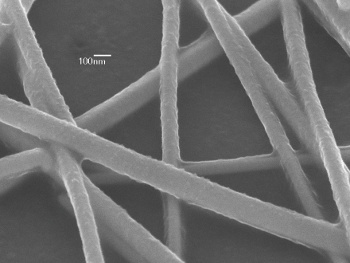 | A scanning-electron micrograph of a silver nanowire transparent conducting film. (UCLA image).[9] |
"In this work, we demonstrate a simple and effective solution method to achieve highly conductive AgNW composite films with excellent optical transparency and mechanical properties... This is by far the best solution: a processed, transparent electrode that is compatible with a wide variety of substrate choices."[9]
References:
- US Geological Survey, Indium - USGS Mineral Resources Program, Mineral Commodity Summaries, 2011.
- Amy C. Tolcin, "Indium," U.S. Department of the Interior, U.S. Geological Survey, Mineral Commodity Summaries 2011, p. 74.
- S.-J. Jiang, Z.-C. Jin, and Claes G. Granqvist, "Low-refractive-index indium-tin-oxyfluoride thin films made by high-rate reactive dc magnetron sputtering," Appl. Opt. vol. 27, no. 14 (July 15, 1988), 2847-2850.
- W. Tanga and D.C. Camerona, "Aluminum-doped zinc oxide transparent conductors deposited by the sol-gel process," Thin Solid Films, vol. 238, no. 1 (January 15, 1994), pp. 83-87.
- Xiaonan Li, Timothy A. Gessert and Timothy Coutts, "The properties of cadmium tin oxide thin-film compounds prepared by linear combinatorial synthesis, Proceedings of the Second Japan-US Workshop on Combinatorial Materials Science and Technology, Applied Surface Science, vol. 223, no. 1-3, February 15, 2004, pp. 138-143.
- Katsuro Hayashi, Satoru Matsuishi, Toshio Kamiya, Masahiro Hirano and Hideo Hosono, "Light-induced conversion of an insulating refractory oxide into a persistent electronic conductor," Nature, vol. 419, no. 6906 (October 3, 2002), pp. 462-465.
- Ivo Jongsma, "Researchers find replacement for rare material indium tin oxide," Eindhoven University of Technology Press Release, April 11. 2011.
- Andriy V. Kyrylyuk, Marie Claire Hermant, Tanja Schilling, Bert Klumperman, Cor E. Koning and Paul van der Schoot, "Controlling electrical percolation in multicomponent carbon nanotube dispersions," Nature Nanotechnology (Published online April 10, 2011)
- Jennifer Marcus, "UCLA team develops highly efficient method for creating flexible, transparent electrodes," UCLA Press Release, November 21, 2011.
- Rui Zhu, Choong-Heui Chung, Kitty C. Cha, Wenbing Yang, Yue Bing Zheng, Huanping Zhou, Tze-Bin Song, Chun-Chao Chen, Paul S. Weiss, Gang Li and Yang Yang, "Fused Silver Nanowires with Metal Oxide Nanoparticles and Organic Polymers for Highly Transparent Conductors," ACS Nano (DOI: 10.1021/nn203576v), October 28, 2011.
- Amy C. Tolcin, "Indium," U.S. Department of the Interior, U.S. Geological Survey, Mineral Commodity Summaries 2011, p. 74.